| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methacrylic acid[1]
| |||
| Preferred IUPAC name
2-Methylprop-2-enoic acid | |||
| Other names
Methacrylic acid
2-Methyl-2-propenoic acid α-Methacrylic acid 2-Methylacrylic acid 2-Methylpropenoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | MAA | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.096 | ||
| EC Number |
| ||
| MeSH | C008384 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
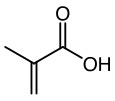
| C4H6O2 | |||
| Molar mass | 86.09 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Acrid, repulsive[2] | ||
| Density | 1.015 g/cm3 | ||
| Melting point | 14 to 15 °C (57 to 59 °F; 287 to 288 K) | ||
| Boiling point | 161 °C (322 °F; 434 K) | ||
| 9% (25 °C)[2] | |||
| Vapor pressure | 0.7 mmHg (20 °C)[2] | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77.2 °C (171.0 °F; 350.3 K) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) [skin][2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 746. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0386". National Institute for Occupational Safety and Health (NIOSH).