| |
| Names | |
|---|---|
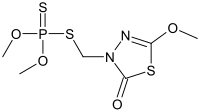
| Preferred IUPAC name
S-[(5-Methoxy-2-oxo-1,3,4-thiadiazol-3(2H)-yl)methyl] O,O-dimethyl phosphorodithioate | |
| Other names
Supracide, Ultracide, Suprathion
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.227 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H11N2O4PS3 | |
| Molar mass | 302.331 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methidathion is an organophosphate insecticide;[1] its use is banned in the European Union and USA.[2]
Methidathion has been used as an insecticide in many countries to control caterpillars of Indarbela quadrinotata.[3]
In 2012, residues were found in Thai vegetables at levels 100 times the legal limit. Thailand routinely uses many pesticides banned in the US and EU and in amounts far exceeding limits.[4]
- ^ Gokalp, Osman; Gulle, Kanat; Sulak, Osman; Cicek, Ekrem; Altuntas, Irfan (2016). "The effects of methidathion on liver: role of vitamins E and C". Toxicology and Industrial Health. 19 (2–6): 63–67. doi:10.1191/0748233703th176oa. PMID 15697176. S2CID 23209774.
- ^ "Archived copy" (PDF). Archived from the original (PDF) on 2013-06-05. Retrieved 2012-03-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ "CONTROL OF BARK EATING CATERPILLAR" (PDF). German Development Cooperation. Archived from the original (PDF) on 13 August 2016. Retrieved 13 July 2016.
- ^ "Nation Thailand news website, thai news, thailand news, Bangkok thailand, aec, breaking news : Nation Thailand".