| IUPAC definition | |
| Micelle | Particle of colloidal dimensions that exists in equilibrium with the molecules or ions in solution from which it is formed.[1][2] |
|---|---|
| Micelle (polymers) | Organized auto-assembly formed in a liquid and composed of amphiphilic macromolecules, in general amphiphilic di- or tri-block copolymers made of solvophilic and solvophobic blocks. |
| Note 1 | An amphiphilic behavior can be observed for water and an organic solvent or between two organic solvents. |
| Note 2 | Polymeric micelles have a much lower critical micellar concentration (CMC) than soap (0.0001 to 0.001 mol/L) or surfactant micelles, but are nevertheless at equilibrium with isolated macromolecules called unimers. Therefore, micelle formation and stability are concentration-dependent.[3] |
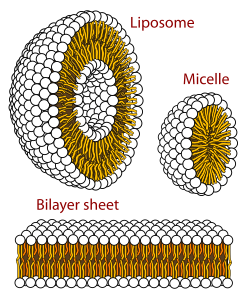
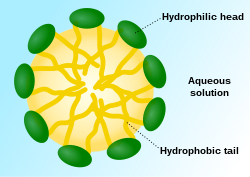
A micelle (/maɪˈsɛl/) or micella (/maɪˈsɛlə/) (pl. micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system).[4] A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.
This phase is caused by the packing behavior of single-tail lipids in a bilayer. The difficulty in filling the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (or oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (or water-in-oil micelle).
Micelles are approximately spherical in shape. Other shapes, such as ellipsoids, cylinders, and bilayers, are also possible. The shape and size of a micelle are a function of the molecular geometry of its surfactant molecules and solution conditions such as surfactant concentration, temperature, pH, and ionic strength. The process of forming micelles is known as micellisation and forms part of the phase behaviour of many lipids according to their polymorphism.[5]
- ^ MacNaugdoesht AD, Wilkinson AR, eds. (1997). Compendium of Chemical Terminology: IUPAC Recommendations (2nd ed.). Oxford: Blackwell Science. ISBN 978-0865426849.
- ^ Slomkowski S, Alemán JV, Gilbert RG, Hess M, Horie K, Jones RG, et al. (2011). "Terminology of polymers and polymerization processes in dispersed systems (IUPAC Recommendations 2011)". Pure and Applied Chemistry. 83 (12): 2229–2259. doi:10.1351/PAC-REC-10-06-03.
- ^ Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P, et al. (2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)". Pure and Applied Chemistry. 84 (2): 377–410. doi:10.1351/PAC-REC-10-12-04.
- ^ "What are Associated Colloids? Given an example". doubtnut.com. Doubtnut. Retrieved 2021-02-26.
- ^ Hamley, I. W. (2007). Introduction to Soft Matter. John Wiley. ISBN 9780470516102.