 | |
| Clinical data | |
|---|---|
| Trade names | Mepact |
| License data | |
| Pregnancy category |
|
| Routes of administration | intravenous liposomal infusion over one hour |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Elimination half-life | minutes (in plasma) 18 hrs (terminal) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
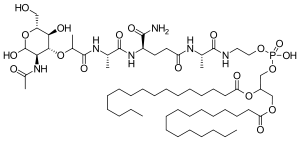
| Formula | C59H109N6O19P |
| Molar mass | 1237.518 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mifamurtide (trade name Mepact, marketed by Takeda) is a drug against osteosarcoma, a kind of bone cancer mainly affecting children and young adults, which is lethal in over half of cases. The drug was approved in Europe in March 2009.