 | |
| Clinical data | |
|---|---|
| Trade names | Cytotec, Misodel, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, vaginal, under the tongue |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | extensively absorbed |
| Protein binding | 80–90% (active metabolite, misoprostol acid) |
| Metabolism | Liver (extensive to misoprostic acid) |
| Elimination half-life | 20–40 minutes |
| Excretion | Urine (80%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.190.521 |
| Chemical and physical data | |
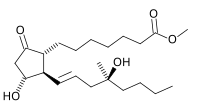
| Formula | C22H38O5 |
| Molar mass | 382.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Misoprostol is a synthetic prostaglandin medication used to prevent and treat stomach and duodenal ulcers, induce labor, cause an abortion, and treat postpartum bleeding due to poor contraction of the uterus.[10][11] It is taken by mouth when used to prevent gastric ulcers in people taking nonsteroidal anti-inflammatory drugs (NSAID).[11] For abortions it is used by itself or in conjunction with mifepristone or methotrexate.[12] By itself, effectiveness for abortion is between 66% and 90%.[13][14] For labor induction or abortion, it is taken by mouth, dissolved in the mouth, or placed in the vagina.[12][15][16][17][18] For postpartum bleeding it may also be used rectally.[19]
Common side effects include diarrhea and abdominal pain.[11] It is in pregnancy category X, meaning that it is known to result in negative outcomes for the fetus if taken during pregnancy.[11] In rare cases, uterine rupture may occur.[11] It is a prostaglandin analogue—specifically, a synthetic prostaglandin E1 (PGE1).[11]
Misoprostol was developed in 1973.[20] It is on the World Health Organization's List of Essential Medicines.[21] It is available as a generic medication.[11]
- ^ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 14 April 2023. Retrieved 14 April 2023.
- ^ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 14 April 2023. Retrieved 14 April 2023.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Angusta (Norgine Pty Ltd)". Therapeutic Goods Administration (TGA). 13 January 2023. Archived from the original on 18 March 2023. Retrieved 9 April 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 15 August 2023.
- ^ "Angusta 25 microgram tablets - Summary of Product Characteristics (SmPC)". (emc). 8 April 2022. Archived from the original on 10 April 2023. Retrieved 9 April 2023.
- ^ "Cytotec- misoprostol tablet". DailyMed. 9 July 2021. Archived from the original on 18 April 2023. Retrieved 13 April 2023.
- ^ "List of nationally authorised medicinal products. Misoprostol (gastrointestinal indication). Procedure no.: PSUSA/00010353/202005" (PDF). European Medicines Agency. 14 January 2021. Archived (PDF) from the original on 29 June 2022. Retrieved 8 August 2021.
- ^ "List of nationally authorised medicinal products. Misoprostol (gastrointestinal indication). Procedure no.: PSUSA/00010291/202006" (PDF). European Medicines Agency. 14 January 2021. Archived (PDF) from the original on 29 June 2022. Retrieved 8 August 2021.
- ^ Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. (2002). "Prevention of NSAID-induced gastroduodenal ulcers". The Cochrane Database of Systematic Reviews. 2011 (4): CD002296. doi:10.1002/14651858.CD002296. PMC 8439413. PMID 12519573. S2CID 1052260.
- ^ a b c d e f g "Misoprostol". The American Society of Health-System Pharmacists. Archived from the original on 21 February 2015. Retrieved 20 February 2015.
- ^ a b Zhang J, Zhou K, Shan D, Luo X (May 2022). "Medical methods for first trimester abortion". The Cochrane Database of Systematic Reviews. 2022 (5): CD002855. doi:10.1002/14651858.CD002855.pub5. PMC 9128719. PMID 35608608.
- ^ Bryant AG, Regan E, Stuart G (January 2014). "An overview of medical abortion for clinical practice". Obstetrical & Gynecological Survey. 69 (1): 39–45. doi:10.1097/OGX.0000000000000017. PMID 25102250. S2CID 28486936.
- ^ Raymond EG, Harrison MS, Weaver MA (January 2019). "Efficacy of Misoprostol Alone for First-Trimester Medical Abortion: A Systematic Review". Obstetrics and Gynecology. 133 (1): 137–147. doi:10.1097/AOG.0000000000003017. PMC 6309472. PMID 30531568.
- ^ Marret H, Simon E, Beucher G, Dreyfus M, Gaudineau A, Vayssière C, et al. (April 2015). "Overview and expert assessment of off-label use of misoprostol in obstetrics and gynaecology: review and report by the Collège national des gynécologues obstétriciens français". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 187: 80–4. doi:10.1016/j.ejogrb.2015.01.018. PMID 25701235.
- ^ Prager S. "Early Pregnancy Loss" (PDF). ACOG Practice Bulletin (200). ACOG. Archived (PDF) from the original on 2 June 2021. Retrieved 2 June 2021.
- ^ Cite error: The named reference
ACOG Practice Bulletin No. 200: Earwas invoked but never defined (see the help page). - ^ "Early Pregnancy Loss". ACOG. 20 January 2015. Archived from the original on 19 January 2023. Retrieved 25 June 2023.
- ^ Blum J, Alfirevic Z, Walraven G, Weeks A, Winikoff B (December 2007). "Treatment of postpartum hemorrhage with misoprostol". International Journal of Gynaecology and Obstetrics. 99 (Suppl 2): S202-5. doi:10.1016/j.ijgo.2007.09.013. PMID 17961565. S2CID 10997666.
- ^ Paul M (2011). "Misoprostol". Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. John Wiley & Sons. ISBN 9781444358476. Archived from the original on 29 June 2022. Retrieved 18 August 2020.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.