| |
| Names | |
|---|---|
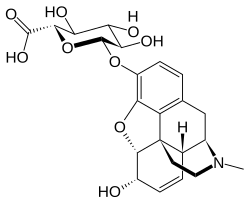
| IUPAC name
6α-Hydroxy-17-methyl-7,8-didehydro-4,5α-epoxymorphinan-3-yl β-D-glucopyranosiduronic acid
| |
| Systematic IUPAC name
(2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-{[(4R,4aR,7S,7aR,12bS)-7-hydroxy-3-methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methano[1]benzofuro[3,2-e]isoquinolin-9-yl]oxy}oxane-2-carboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| MeSH | Morphine-3-glucuronide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H27NO9 | |
| Molar mass | 461.462 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Morphine-3-glucuronide is a metabolite of morphine produced by UGT2B7.[1] It is not active as an opioid agonist,[2] but does have some action as a convulsant, which does not appear to be mediated through opioid receptors,[3] but rather through interaction with glycine and/or GABA receptors. As a polar compound, it has a limited ability to cross the blood–brain barrier, but kidney failure may lead to its accumulation and result in seizures. Probenecid and inhibitors of P-glycoprotein can enhance uptake of morphine-3-glucuronide and, to a lesser extent, morphine-6-glucuronide.[4][page needed] Reported side effects related to the accumulation of this metabolite include convulsions, agitation, hallucinations, hyperalgesia, and coma.
- ^ Coffman BL, Rios GR, King CD, Tephly TR (1 January 1997). "Human UGT2B7 catalyzes morphine glucuronidation". Drug Metab. Dispos. 25 (1): 1–4. PMID 9010622.
- ^ Vindenes V, Ripel A, Handal M, Boix F, Mørland J (July 2009). "Interactions between morphine and the morphine-glucuronides measured by conditioned place preference and locomotor activity". Pharmacology Biochemistry and Behavior. 93 (1): 1–9. doi:10.1016/j.pbb.2009.03.013. PMID 19351545. S2CID 5328449.
- ^ Hemstapat K, Le L, Edwards SR, Smith MT (July 2009). "Comparative studies of the neuro-excitatory behavioural effects of morphine-3-glucuronide and dynorphin a(2-17) following spinal and supraspinal routes of administration". Pharmacology Biochemistry and Behavior. 93 (4): 498–505. doi:10.1016/j.pbb.2009.06.016. PMID 19580825. S2CID 207331030.
- ^ Bertram G. Katzung; Susan B. Masters; Anthony J. Trevor. Basic & Clinical Pharmacology (11th ed.).