 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Durabolin, others |
| Other names | • NPP • Nandrolone phenpropionate • 19-Nortestosterone phenylpropionate • Nandrolone hydrocinnamate • 19-Nortestosterone 17β-phenylpropionate • NSC-23162 |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | • Oral: 0.3–2.9% (pigs)[1] • Intramuscular: high[2] |
| Metabolism | Blood (hydrolysis), liver (reduction)[7][5] |
| Metabolites | • Nandrolone[3] • 5α-Dihydronandrolone[3] • 19-Norandrosterone[4] • 19-Noretiocholanolone[4] • Conjugates[5] |
| Elimination half-life | • Intramuscular: 2.7 days[6] • Nandrolone: <4.3 hours[7] |
| Duration of action | • Intramuscular: 5–7 days[3][6] |
| Excretion | Urine[7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.502 |
| Chemical and physical data | |
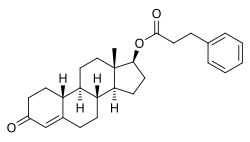
| Formula | C27H34O3 |
| Molar mass | 406.566 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nandrolone phenylpropionate (NPP), or nandrolone phenpropionate, sold under the brand name Durabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used primarily in the treatment of breast cancer and osteoporosis in women.[8][9][10][11][3] It is given by injection into muscle once every week.[3] Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly no longer available.[3][11]
Side effects of NPP include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[3] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[3][12] It has strong anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women and children.[3][12][13] NPP is a nandrolone ester and a long-lasting prodrug of nandrolone in the body.[3]
NPP was first described in 1957 and was introduced for medical use in 1959.[3] It was the first nandrolone ester to be introduced, followed by nandrolone decanoate in 1962, and has been one of the most widely used nandrolone esters.[3][14] However, in more recent times, the drug has been largely superseded by nandrolone decanoate, which is longer-acting and more convenient to use.[3][11] In addition to its medical use, NPP is used to improve physique and performance.[3] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[3]
- ^ McEvoy JD, McVeigh CE, McCaughey WJ (December 1998). "Residues of nortestosterone esters at injection sites. Part 1. Oral bioavailability". The Analyst. 123 (12): 2475–2478. doi:10.1039/a804919j. PMID 10435281.
- ^ Matsumoto AM (2001). "Clinical Use and Abuse of Androgens and Antiandrogens". In Becker KL (ed.). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185–. ISBN 978-0-7817-1750-2.
- ^ a b c d e f g h i j k l m n o William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 460–467, 193–194. ISBN 978-0-9828280-1-4.
- ^ a b Rogozkin VA (14 June 1991). "Enzymic Systems Participating in AAS Metabolism". Metabolism of Anabolic-Androgenic Steroids. CRC Press. pp. 108–. ISBN 978-0-8493-6415-0.
- ^ a b Colby HD, Longhurst PA (6 December 2012). "Fate of Anabolic Steroids in the Body". In Thomas JA (ed.). Drugs, Athletes, and Physical Performance. Springer Science & Business Media. pp. 27–29. ISBN 978-1-4684-5499-4.
- ^ a b Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ (April 1997). "Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume". The Journal of Pharmacology and Experimental Therapeutics. 281 (1): 93–102. PMID 9103484.
- ^ a b c "Decadurabolin injection Data Sheet" (PDF). Merck Sharp & Dohme (NZ) Ltd. Archived from the original (PDF) on 21 January 2015. Retrieved 2017-12-13.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 660–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–717. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- ^ a b c "Nandrolone - FDA prescribing information, side effects and uses".
- ^ a b Kicman AT (June 2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ Potts GO, Arnold A, Beyler AL (6 December 2012). "Dissociation of the Androgenic and Other Hormonal Activities from the Protein Anabolic Effects of Steroids". In Kochakian CD (ed.). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 401–. ISBN 978-3-642-66353-6.
- ^ Sneader W (23 June 2005). "Drugs from Naturally Occurring Prototypes: Biochemicals". Drug Discovery: A History. John Wiley & Sons. pp. 206–. ISBN 978-0-471-89979-2.