Nanofluidics is the study of the behavior, manipulation, and control of fluids that are confined to structures of nanometer (typically 1–100 nm) characteristic dimensions (1 nm = 10−9 m). Fluids confined in these structures exhibit physical behaviors not observed in larger structures, such as those of micrometer dimensions and above, because the characteristic physical scaling lengths of the fluid, (e.g. Debye length, hydrodynamic radius) very closely coincide with the dimensions of the nanostructure itself.
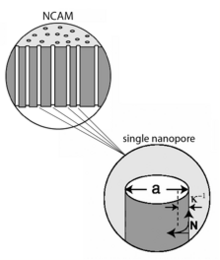
When structures approach the size regime corresponding to molecular scaling lengths, new physical constraints are placed on the behavior of the fluid. For example, these physical constraints induce regions of the fluid to exhibit new properties not observed in bulk, e.g. vastly increased viscosity near the pore wall; they may effect changes in thermodynamic properties and may also alter the chemical reactivity of species at the fluid-solid interface. A particularly relevant and useful example is displayed by electrolyte solutions confined in nanopores that contain surface charges, i.e. at electrified interfaces, as shown in the nanocapillary array membrane (NCAM) in the accompanying figure.
All electrified interfaces induce an organized charge distribution near the surface known as the electrical double layer. In pores of nanometer dimensions the electrical double layer may completely span the width of the nanopore, resulting in dramatic changes in the composition of the fluid and the related properties of fluid motion in the structure. For example, the drastically enhanced surface-to-volume ratio of the pore results in a preponderance of counter-ions (i.e. ions charged oppositely to the static wall charges) over co-ions (possessing the same sign as the wall charges), in many cases to the near-complete exclusion of co-ions, such that only one ionic species exists in the pore. This can be used for manipulation of species with selective polarity along the pore length to achieve unusual fluidic manipulation schemes not possible in micrometer and larger structures.