| |

| |
| Names | |
|---|---|
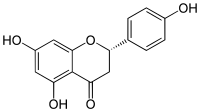
| IUPAC name
(2S)-4′,5,7-Trihydroxyflavan-4-one
| |
| Systematic IUPAC name
(2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Naringetol; Salipurol; Salipurpol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.865 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H12O5 | |
| Molar mass | 272.256 g·mol−1 |
| Melting point | 251 °C (484 °F; 524 K)[1] |
| 475 mg/L[citation needed] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Naringenin is a flavanone from the flavonoid group of polyphenols.[2] It is commonly found in citrus fruits, especially as the predominant flavonone in grapefruit.[2]
The fate and biological functions of naringenin in vivo are unknown, remaining under preliminary research, as of 2024.[2] High consumption of dietary naringenin is generally regarded as safe, mainly due to its low bioavailability.[2] Taking dietary supplements or consuming grapefruit excessively may impair the action of anticoagulants and increase the toxicity of various prescription drugs.[2]
Similar to furanocoumarins present in citrus fruits, naringenin may evoke CYP3A4 suppression in the liver and intestines, possibly resulting in adverse interactions with common medications.[2][3][4][5]
- ^ Naringenin at the Human Metabolome Database
- ^ a b c d e f "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2024. Retrieved 9 May 2024.
- ^ Lohezic-Le Devehat, F.; Marigny, K.; Doucet, M.; Javaudin, L. (2002). "[Grapefruit juice and drugs: a hazardous combination?]". Therapie. 57 (5): 432–445. ISSN 0040-5957. PMID 12611197.
- ^ Singh, B. N. (September 1999). "Effects of food on clinical pharmacokinetics". Clinical Pharmacokinetics. 37 (3): 213–255. doi:10.2165/00003088-199937030-00003. ISSN 0312-5963. PMID 10511919.
- ^ Fuhr, U. (April 1998). "Drug interactions with grapefruit juice. Extent, probable mechanism and clinical relevance". Drug Safety. 18 (4): 251–272. doi:10.2165/00002018-199818040-00002. ISSN 0114-5916. PMID 9565737.