 | |
| Clinical data | |
|---|---|
| Trade names | Nevanac, Ilevro, Amnac, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606007 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.414 |
| Chemical and physical data | |
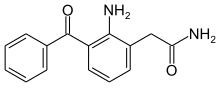
| Formula | C15H14N2O2 |
| Molar mass | 254.289 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nepafenac, sold under the brand name Nevanac among others, is a nonsteroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop 0.1% solution (Nevanac) or 0.3% solution (Ilevro). It is used to treat pain and inflammation associated with cataract surgery.[3] Nepafenac is a prodrug of amfenac, an inhibitor of COX-1 and COX-2 activity.[4][5]
- ^ "Nepafenac ophthalmic Use During Pregnancy". Drugs.com. 6 June 2019. Retrieved 13 September 2020.
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2015". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ Nepafenac Monograph
- ^ Drugbank: Nepafenac
- ^ Zanetti FR, Fulco EA, Chaves FR, da Costa Pinto AP, Arieta CE, Lira RP (July 2012). "Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial". Indian Journal of Ophthalmology. 60 (4): 277–81. doi:10.4103/0301-4738.98705. PMC 3442462. PMID 22824596.