
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
Nickel(2+) diacetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.006.147 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6NiO4 | |
| Molar mass | 176.781 g·mol−1 |
| Appearance | Mint-green Solid |
| Odor | slight acetic acid |
| Density | 1.798 g/cm3 (anhydrous) 1.744 g/cm3 (tetrahydrate) |
| Melting point | decomposes when heated [1][2] |
| Easily soluble in cold water, hot water | |
| Solubility | Soluble in methanol insoluble in diethyl ether, n-octanol |
| +4,690.0·10−6 cm3/mol | |
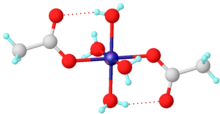
| Structure | |
| monoclinic | |
| P21/c | |
a = 4.764, b = 11.771, c = 8.425 Å α = 90°, β = 93.6°, γ = 90°[3] tetrahydrate
| |
Lattice volume (V)
|
471.5 |
Formula units (Z)
|
2 |
| distorted octahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
350 mg/kg (rat, oral) 410 mg/kg (mouse, oral)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The mint-green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.
- ^ M. A. Mohamed, S. A. Halawy, M. M. Ebrahim: "Non-isothermal decomposition of nickel acetate tetrahydrate", in: Journal of Analytical and Applied Pyrolysis, 1993, 27 (2), S. 109–110. doi:10.1016/0165-2370(93)80002-H.
- ^ G. A. M. Hussein, A. K. H. Nohman, K. M. A. Attyia: "Characterization of the decomposition course of nickel acetate tetrahydrate in air", in: Journal of Thermal Analysis and Calorimetry, 1994, 42, S. 1155–1165; doi:10.1007/BF02546925.
- ^ Downie, T. C.; Harrison, W.; Raper, E. S.; Hepworth, M. A. (15 March 1971). "A three-dimensional study of the crystal structure of nickel acetate tetrahydrate". Acta Crystallographica Section B. 27 (3): 706–712. Bibcode:1971AcCrB..27..706D. doi:10.1107/S0567740871002802.
- ^ "Nickel metal and other compounds (as Ni)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
