| |

| |
| Names | |
|---|---|
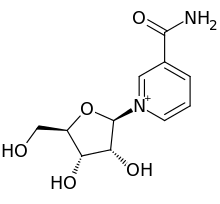
| IUPAC name
3-Carbamoyl-1-(β-D-ribofuranosyl)pyridin-1-ium
| |
| Systematic IUPAC name
3-Carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium | |
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H15N2O5+ | |
| Molar mass | 255.25 g/mol |
| Melting point | Complete thermal degradation occurs above 130°C (chloride salt)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nicotinamide riboside (NR, SR647) is a pyridine-nucleoside and a form of vitamin B3. It functions as a precursor to nicotinamide adenine dinucleotide, or NAD+,[2] through a two-step and a three-step pathway.[3]
- ^ Campbell, MT; Jones, DS; Andrews, GP; Li, S (2019). "Understanding the physicochemical properties and degradation kinetics of nicotinamide riboside chloride" (PDF). Food & Nutrition Research. 63: 3419. doi:10.29219/fnr.v63.3419.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bogan, KL; Brenner, C (2008). "Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition". Annu. Rev. Nutr. 28: 115–130. doi:10.1146/annurev.nutr.28.061807.155443. PMID 18429699.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mehmel, M; Jovanović, N; Spitz, U (31 May 2020). "Nicotinamide Riboside-The Current State of Research and Therapeutic Uses". Nutrients. 12 (6): 1616. doi:10.3390/nu12061616. PMC 7352172. PMID 32486488.