This article needs more reliable medical references for verification or relies too heavily on primary sources. (July 2024) |  |
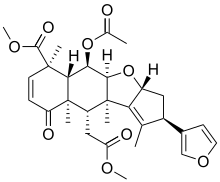
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (2R,3aR,4aS,5R,5aR,6R,9aR,10S,10aR)-5-(acetyloxy)-2-(furan-3-yl)-10-(2-methoxy-2-oxoethyl)-1,6,9a,10a-tetramethyl-9-oxo-3,3a,4a,5,5a,6,9,9a,10,10a-decahydro-2H-cyclopenta[b]naphtho[2,3-d]furan-6-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.106.899 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H36O9 | |
| Molar mass | 540.609 g·mol−1 |
| Melting point | 205 °C (401 °F; 478 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Nimbin is a triterpenoid isolated from the neem tree (Azadirachta indica). Nimbin is thought to be responsible for much of the biological activities of neem oil, and is reported to have anti-inflammatory, antipyretic, fungicidal, antihistamine and antiseptic properties.[2] The neem tree is found in multiple Asian countries such as China, Thailand, and India. Nimbin is part of the chemical family of limonoids and triterpenoids. Nimbin was first extracted in 1942 from neem seeds by Siddiqi et al.[citation needed] Its molecular formula was established by mass-spectrometry along with salannin, a compound whose chemical formula and properties are very close those of nimbin. Nimbin can be extracted from different parts of the neem tree with a solvent or supercritical carbon dioxide.[3][4] Nimbin is used for different purposes because it has multiple properties such as insecticide,[5][6] antiviral, antimicrobial,[7] anti-inflammatory,[8] and anti-fungal.[9] Nimbin was commonly used in traditional Indian and Chinese medicine. For example, it can be used to treat skin conditions like eczema and psoriasis.[medical citation needed]
Studies have also shown that it can be used to treat diseases caused by viruses such as the SARS COV-2[10][11] or the dengue virus.[12][13] However, that hasn't been demonstrated in humans and only in laboratory settings. It was a derivative of nimbin (named N2) that was used in laboratories for the dengue virus and other uses like antimicrobial. Nimbin is relatively hydrophobic,[14] and there has been a study to make it more hydrophilic with an inclusion complex which can be helpful to enable its direct use.

- ^ Siddiqui, Salimuzzaman (1945). "Utilization of nim oil and its bitter constituents (nimbidin series) in the pharmaceutical industry". Journal of Scientific & Industrial Research. 4: 5–10.
- ^ W. Kraus, "Biologically active ingredients-azadirachtin and other triterpenoids", in: H. Schutterer (Ed.), The Neem Tree Azadirachta indica A. Juss and Other Meliaceous Plants, Weinheim, New York, 1995, p 35-88
- ^ Cite error: The named reference
Optimization-Supercritical-Extractionwas invoked but never defined (see the help page). - ^ Tonthubthimthong, Pathumthip; Douglas, Peter L.; Douglas, Supaporn; Luewisutthichat, Wilai; Teppaitoon, Wittaya; Pengsopa, La-eid (2004-08-01). "Extraction of nimbin from neem seeds using supercritical CO2 and a supercritical CO2–methanol mixture". The Journal of Supercritical Fluids. 30 (3): 287–301. doi:10.1016/j.supflu.2003.07.007. ISSN 0896-8446.
- ^ Agasimundin, Varalaxmi B.; Rangiah, Kannan; Sheetal, Ambardar; Gowda, Malali (2019), Gowda, Malali; Sheetal, Ambardar; Kole, Chittaranjan (eds.), "Neem Microbiome", The Neem Genome, Compendium of Plant Genomes, Cham: Springer International Publishing, pp. 111–123, doi:10.1007/978-3-030-16122-4_12, ISBN 978-3-030-16121-7, S2CID 239213089, retrieved 2023-03-21
- ^ Simmonds, Monique SJ; Jarvis, Andrew P; Johnson, Shaun; Jones, Graeme R; Morgan, E David (2004-04-27). "Comparison of anti-feedant and insecticidal activity of nimbin and salannin photo-oxidation products with neem(Azadirachta indica) limonoids". Pest Management Science. 60 (5): 459–464. doi:10.1002/ps.834. ISSN 1526-498X. PMID 15154512.
- ^ Coventry, E.; Allan, E. J. (2001). "Microbiological and Chemical Analysis of Neem (Azadirachta indica) Extracts: New Data on Antimicrobial Activity". Phytoparasitica. 29 (5): 441–450. Bibcode:2001Phyto..29..441C. doi:10.1007/BF02981863. ISSN 0334-2123. S2CID 5618875.
- ^ Sudhakaran, Gokul; Prathap, Pandurangan; Guru, Ajay; Rajesh, Ravi; Sathish, Sruthy; Madhavan, Thirumurthy; Arasu, Mariadhas V.; Al-Dhabi, Naif A.; Choi, Ki Choon; Gopinath, Pushparathinam; Arockiaraj, Jesu (2022-02-03). "Anti-inflammatory role demonstrated both in vitro and in vivo models using nonsteroidal tetranortriterpenoid, Nimbin (N1) and its analogs (N2 and N3) that alleviate the domestication of alternative medicine". Cell Biology International. 46 (5): 771–791. doi:10.1002/cbin.11769. ISSN 1065-6995. PMID 35077598. S2CID 246287761.
- ^ Cite error: The named reference
:122was invoked but never defined (see the help page). - ^ Maurya, Vimal K.; Kumar, Swatantra; Prasad, Anil K.; Bhatt, Madan L. B.; Saxena, Shailendra K. (2020-05-24). "Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor". VirusDisease. 31 (2): 179–193. doi:10.1007/s13337-020-00598-8. ISSN 2347-3584. PMC 7245990. PMID 32656311. S2CID 256093325.
- ^ Sarkar, Lucky; Oko, Lauren; Gupta, Soham; Bubak, Andrew N.; Das, Bishnu; Gupta, Parna; Safiriyu, Abass Alao; Singhal, Chirag; Neogi, Ujjwal; Bloom, David; Banerjee, Arup; Mahalingam, Ravi; Cohrs, Randall J.; Koval, Michael; Shindler, Kenneth S. (2022-01-12). "Azadirachta indica A. Juss bark extract and its Nimbin isomers restrict β-coronaviral infection and replication". Virology. 569: 13–28. doi:10.1016/j.virol.2022.01.002. ISSN 0042-6822. PMC 8844965. PMID 35219218.
- ^ Yildiz, M.; Hardy, J.A. (2013-11-27). "NS2B-NS3 protease from dengue virus at pH 8.5". doi:10.2210/pdb4m9m/pdb. Retrieved 2023-03-23.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Lavanya, P.; Ramaiah, Sudha; Anbarasu, Anand (2015-11-20). "Computational analysis reveal inhibitory action of nimbin against dengue viral envelope protein". VirusDisease. 26 (4): 243–254. doi:10.1007/s13337-015-0280-x. ISSN 2347-3584. PMC 4663709. PMID 26645034.
- ^ Yang, Li-Juan; Yang, Bo; Chen, Wen; Huang, Rong; Yan, Sheng-Jiao; Lin, Jun (2010-07-20). "Host−Guest System of Nimbin and β-Cyclodextrin or Its Derivatives: Preparation, Characterization, Inclusion Mode, and Solubilization". Journal of Agricultural and Food Chemistry. 58 (15): 8545–8552. doi:10.1021/jf101079e. ISSN 0021-8561. PMID 20681641.