| |
| Names | |
|---|---|
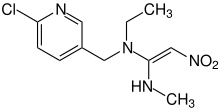
| Preferred IUPAC name
(E)-N1-[(6-Chloropyridin-3-yl)methyl]-N1-ethyl-N′1-methyl-2-nitroethene-1,1-diamine | |
| Other names
Capstar
| |
| Identifiers | |
3D model (JSmol)
|
|
| 8489488 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.162.838 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H15ClN4O2 | |
| Molar mass | 270.72 g/mol |
| Appearance | Pale yellow crystalline solid |
| Density | 1.4 (g/mL) |
| Melting point | 82 °C (180 °F; 355 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302 | |
| P264, P270, P301+P312, P330, P501 | |
| Pharmacology | |
| QP53BX02 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitenpyram is a chemical frequently used as an insecticide in agriculture and veterinary medicine. The compound is an insect neurotoxin belonging to the class of neonicotinoids which works by blocking neural signaling of the central nervous system. It does so by binding irreversibly to the nicotinic acetylcholine receptor (nACHr) causing a stop of the flow of ions in the postsynaptic membrane of neurons leading to paralysis and death. Nitenpyram is highly selective towards the variation of the nACHr which insects possess, and has seen extensive use in targeted, insecticide applications.
Known under the codename TI 304 during field testing starting in 1989, the compound's first documented commercial use was in 1995 under the name "Bestguard" as an agricultural insecticide.[1] Later, nitenpyram was expanded for use as a flea treatment by the Novartis company under the trade name "Capstar", with a subsequent FDA approval for non-food producing animals in October 2000. The current producer of nitenpyram itself is the Sumitomo chemical company. Nitenpyram continues to be used commercially, though data from market surveys indicate a significant decrease in the global usage compared to other insecticides or neonicotinoids.[2]
Due to its use as an insecticide and treatment of non-food producing animals, it was not deemed necessary to research the human toxicology during its main use, and, as such, not much is known about the details of nitenpyram's effects on humans. Looking at rat experiments however, the lethal amount of nitenpyram is quite high (on the order of grams) in mammals in general, whereas invertebrates will die with only micro or nanograms of the substance.[3][4]
Neonicotinoids, in general, have a low degradation rate when used for agricultural purposes, which allows for long-lasting protection of the crops against plant-sucking insects and indirectly the plant diseases these insects might carry.[1]
- ^ a b Yamamoto, I.; Casida, J.E (1999). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor | SpringerLink. doi:10.1007/978-4-431-67933-2. ISBN 978-4-431-68011-6. S2CID 34374399.
- ^ Pisa, Lennard; Goulson, Dave; Yang, En-Cheng; Gibbons, David; Sánchez-Bayo, Francisco; Mitchell, Edward; Aebi, Alexandre; Sluijs, Jeroen van der; MacQuarrie, Chris J. K. (2017). "An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: impacts on organisms and ecosystems". Environmental Science and Pollution Research. 28 (10): 11749–11797. doi:10.1007/s11356-017-0341-3. PMC 7921077. PMID 29124633.
- ^ "ChemSpider | Data Source Details | Oxford University Chemical Safety Data (No longer updated)". www.chemspider.com. Retrieved 2018-03-21.
- ^ Pubchem. "Nitenpyram". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-03-21.