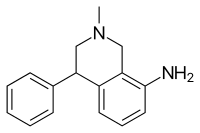
 | |
| Clinical data | |
|---|---|
| Trade names | Merital |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 1.5–4 hours |
| Excretion | Kidney (88%) within 24 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H18N2 |
| Molar mass | 238.334 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nomifensine, sold under the brand names Merital and Alival, is a norepinephrine–dopamine reuptake inhibitor (NDRI), i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters.[3] This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.[4]
The drug was developed in the 1960s by Hoechst AG (now Sanofi-Aventis),[5] who then test marketed it in the United States. It was an effective antidepressant, without sedative effects. Nomifensine did not interact significantly with alcohol and lacked anticholinergic effects. No withdrawal symptoms were seen after 6 months treatment. The drug was however considered not suitable for agitated patients as it presumably made agitation worse.[6][7] In January 1986 the drug was withdrawn by its manufacturers for safety reasons.[8]
Some case reports in the 1980s suggested that there was potential for psychological dependence on nomifensine, typically in patients with a history of stimulant addiction, or when the drug was used in very high doses (400–600 mg per day).[9]
In a 1989 study it was investigated for use in treating adult ADHD and proven effective.[10] In a 1977 study it was not proven of benefit in advanced parkinsonism, except for depression associated with the parkinsonism.[11]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Heptner W, Hornke I, Uihlein M (April 1984). "Kinetics and metabolism of nomifensine". The Journal of Clinical Psychiatry. 45 (4 Pt 2): 21–5. PMID 6370971.
- ^ Brogden RN, Heel RC, Speight TM, Avery GS (July 1979). "Nomifensine: A review of its pharmacological properties and therapeutic efficacy in depressive illness". Drugs. 18 (1): 1–24. doi:10.2165/00003495-197918010-00001. PMID 477572. S2CID 23952170.
- ^ 'Chirality and Biological Activity of Drugs' page 138
- ^ US patent 3577424, Ehrhart G, Schmitt K, Hoffmann I, Ott H, "4-Phenyl-8-Amino Tetrahydroisoquinolines", issued 1971-05-04, assigned to Farbwerke Hoechst
- ^ Habermann W (1977). "A review of controlled studies with nomifensine, performed outside the UK". British Journal of Clinical Pharmacology. 4 (Suppl 2): 237S–241S. doi:10.1111/j.1365-2125.1977.tb05759.x. PMC 1429098. PMID 334230.
- ^ Yakabow AL, Hardiman S, Nash RJ (April 1984). "An overview of side effects and long-term experience with nomifensine from United States clinical trials". The Journal of Clinical Psychiatry. 45 (4 Pt 2): 96–101. PMID 6370985.
- ^ "CSM Update: Withdrawal of nomifensine". British Medical Journal. 293 (6538): 41. July 1986. doi:10.1136/bmj.293.6538.41. PMC 1340782. PMID 20742679.
- ^ Böning J, Fuchs G (September 1986). "Nomifensine and psychological dependence--a case report". Pharmacopsychiatry. 19 (5): 386–8. doi:10.1055/s-2007-1017275. PMID 3774872. S2CID 29192368.
- ^ Shekim WO, Masterson A, Cantwell DP, Hanna GL, McCracken JT (May 1989). "Nomifensine maleate in adult attention deficit disorder". The Journal of Nervous and Mental Disease. 177 (5): 296–9. doi:10.1097/00005053-198905000-00008. PMID 2651559. S2CID 1932119.
- ^ Bedard P, Parkes JD, Marsden CD (1977). "Nomifensine in Parkinson's disease". British Journal of Clinical Pharmacology. 4 (Suppl 2): 187S–190S. doi:10.1111/j.1365-2125.1977.tb05751.x. PMC 1429119. PMID 334223.