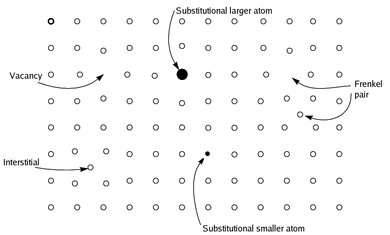
Non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work.[not verified in body]
Contrary to earlier definitions, modern understanding of non-stoichiometric compounds view them as homogeneous, and not mixtures of stoichiometric chemical compounds.[not verified in body] Since the solids are overall electrically neutral, the defect is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. Many metal oxides and sulfides have non-stoichiometric examples; for example, stoichiometric iron(II) oxide, which is rare, has the formula FeO, whereas the more common material is nonstoichiometric, with the formula Fe0.95O. The type of equilibrium defects in non-stoichiometric compounds can vary with attendant variation in bulk properties of the material.[1] Non-stoichiometric compounds also exhibit special electrical or chemical properties because of the defects; for example, when atoms are missing, electrons can move through the solid more rapidly.[not verified in body] Non-stoichiometric compounds have applications in ceramic and superconductive material and in electrochemical (i.e., battery) system designs.[citation needed]
- ^ Geng, Hua Y.; et al. (2012). "Anomalies in nonstoichiometric uranium dioxide induced by a pseudo phase transition of point defects". Phys. Rev. B. 85 (14): 144111. arXiv:1204.4607. Bibcode:2012PhRvB..85n4111G. doi:10.1103/PhysRevB.85.144111. S2CID 119288531.