 | |
| Clinical data | |
|---|---|
| Addiction liability | High |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.161.803 |
| Chemical and physical data | |
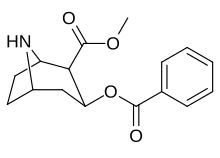
| Formula | C16H19NO4 |
| Molar mass | 289.331 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine,[1] although salicylmethylecgonine is also speculated to be an active metabolite. The local anesthetic potential of norcocaine has been shown to be higher than that of cocaine,[2][3] however cocaine continues to be more widely used. Norcocaine used for research purposes is typically synthesized from cocaine. Several methods for the synthesis have been described.[4][5]
- ^ "Virtual Mass Spectrometry Laboratory: Cocaine in Hair". Archived from the original on 2007-09-01. Retrieved 2008-01-14.
- ^ Wang Q, Simpao A, Sun L, Falk JL, Lau CE (January 2001). "Contribution of the active metabolite, norcocaine, to cocaine's effects after intravenous and oral administration in rats: pharmacodynamics". Psychopharmacology. 153 (3): 341–52. doi:10.1007/s002130000568. PMID 11271407. S2CID 10708670.
- ^ Just WW, Hoyer J (January 1977). "The local anesthetic potency of norcocaine, a metabolite of cocaine". Experientia. 33 (1). Birkhäuser: 70–1. doi:10.1007/BF01936761. PMID 836425. S2CID 32483810.
- ^ Stenberg VI, Narain NK, Singh SP, Parmar SS (April 1976). "An improved synthesis of norcocaine". Journal of Heterocyclic Chemistry. 13 (2): 363–364. doi:10.1002/jhet.5570130231.
- ^ Lazer ES, Aggarwal ND, Hite GJ, Nieforth KA, Kelleher RT, Spealman RD, et al. (December 1978). "Synthesis and biological activity of cocaine analogs I: N-alkylated norcocaine derivatives". Journal of Pharmaceutical Sciences. 67 (12): 1656–8. doi:10.1002/jps.2600671204. PMID 102759.