| |
| Names | |
|---|---|
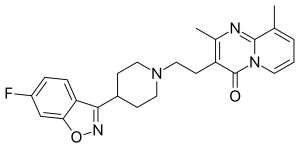
| Preferred IUPAC name
3-{2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2,9-dimethyl-4H-pyrido[1,2-a]pyrimidin-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
| MeSH | C072259 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H25FN4O2 | |
| Molar mass | 420.488 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ocaperidone (R 79598) is a benzisoxazole antipsychotic.[1] It was initially developed by Janssen, later licensed to French laboratory Neuro3D and then acquired in 2007 by German company Evotec. It was found to be more potent than risperidone in animal studies,[2] but its testing was abandoned in 2010 after unfavorable results in human Phase II trials,[3] as while it was effective at controlling symptoms of schizophrenia, ocaperidone produced an unacceptable amount of extrapyramidal side effects.[4]
- ^ Leysen, JE; Janssen, PM; Gommeren, W; Wynants, J; Pauwels, PJ; Janssen, PA (1992). "In vitro and in vivo receptor binding and effects on monoamine turnover in rat brain regions of the novel antipsychotics risperidone and ocaperidone". Molecular Pharmacology. 41 (3): 494–508. PMID 1372084.
- ^ Megens AA, Awouters FH, Meert TF, Schellekens KH, Niemegeers CJ, Janssen PA. Pharmacological profile of the new potent neuroleptic ocaperidone (R 79,598). J Pharmacol Exp Ther. 1992 Jan;260(1):146-59. PMID 1370538
- ^ "Ocaperidone — AdisInsight". Adis Insight. Adis International Ltd, part of Springer Science+Business Media. Retrieved 10 December 2015.
- ^ Geerts, Hugo; Spiros, Athan; Roberts, Patrick; Twyman, Roy; Alphs, Larry; Grace, Anthony A. (2012). "Blinded Prospective Evaluation of Computer-Based Mechanistic Schizophrenia Disease Model for Predicting Drug Response". PLOS ONE. 7 (12): e49732. Bibcode:2012PLoSO...749732G. doi:10.1371/journal.pone.0049732. PMC 3522663. PMID 23251349.