 | |
| Clinical data | |
|---|---|
| Pronunciation | OH li SER i deen |
| Trade names | Olinvyk |
| Other names | TRV-130, TRV130 |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | Intravenous[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
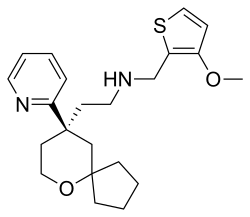
| Formula | C22H30N2O2S |
| Molar mass | 386.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Oliceridine, sold under the brand name Olinvyk, is an opioid medication that is used for the treatment of moderate to severe acute pain in adults.[3] It is given by intravenous (IV) injection.[3]
The most common side effects include nausea, vomiting, dizziness, headache, constipation, itchy skin and low oxygen levels in blood.[4]
It was approved for medical use in the United States in August 2020.[4]
- ^ "Olinvyk- oliceridine injection, solution". DailyMed. 18 August 2020. Retrieved 16 September 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b "FDA Approves New Opioid for Intravenous Use in Hospitals, Other Controlled Clinical Settings". U.S. Food and Drug Administration (FDA) (Press release). 7 August 2020. Retrieved 7 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Drug Trials Snapshots: Olinvyk". U.S. Food and Drug Administration (FDA). 7 August 2020. Retrieved 16 September 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.