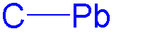
Organolead chemistry is the scientific study of the synthesis and properties of organolead compounds, which are organometallic compounds containing a chemical bond between carbon and lead. The first organolead compound was hexaethyldilead (Pb2(C2H5)6), first synthesized in 1858.[1] Sharing the same group with carbon, lead is tetravalent.
Going down the carbon group the C–X (X = C, Si, Ge, Sn, Pb) bond becomes weaker and the bond length larger. The C–Pb bond in tetramethyllead is 222 pm long with a dissociation energy of 49 kcal/mol (204 kJ/mol). For comparison the C–Sn bond in tetramethyltin is 214 pm long with dissociation energy 71 kcal/mol (297 kJ/mol). The dominance of Pb(IV) in organolead chemistry is remarkable because inorganic lead compounds tend to have Pb(II) centers. The reason is that with inorganic lead compounds elements such as nitrogen, oxygen and the halides have a much higher electronegativity than lead itself and the partial positive charge on lead then leads to a stronger contraction of the 6s orbital than the 6p orbital making the 6s orbital inert; this is called the inert-pair effect.[2]
By far the organolead compound that has had the greatest impact is tetraethyllead, formerly used as an antiknock agent in gasoline intended for automobile internal combustion engines and still widely used in avgas for small aircraft.[3] The most important lead reagents for introducing lead are lead tetraacetate and lead(II) chloride.
The use of organoleads is limited partly due to their toxicity.
- ^ Main Group Metals in Organic Synthesis Yamamoto, Hisashi / Oshima, Koichiro (eds.) 2004 ISBN 3-527-30508-4
- ^ Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. 1997
- ^ "When will we see unleaded AvGas?". Retrieved 2024-05-26.