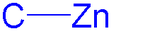
Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.[1][2][3][4]
Organozinc compounds were among the first organometallic compounds made. They are less reactive than many other analogous organometallic reagents, such as Grignard and organolithium reagents. In 1848 Edward Frankland prepared the first organozinc compound, diethylzinc, by heating ethyl iodide in the presence of zinc metal.[5] This reaction produced a volatile colorless liquid that spontaneous combusted upon contact with air. Due to their pyrophoric nature, organozinc compounds are generally prepared using air-free techniques. They are unstable toward protic solvents. For many purposes they are prepared in situ, not isolated, but many have been isolated as pure substances and thoroughly characterized.[6]
Organozincs can be categorized according to the number of carbon substituents that are bound to the metal.[2][3]
- Diorganozinc (R2Zn): A class of organozinc compounds in which two alkyl ligands. These may be further divided into subclasses depending on the other ligands attached
- Heteroleptic (RZnX): Compounds which an electronegative or monoanionic ligand (X), such as a halide, is attached to the zinc center with another alkyl or aryl substituent (R).
- Ionic organozinc compounds: This class is divided into organozincates (RnZn−) and organozinc cations (RZnL+
n).
- ^ Knochel, Paul; Millot, Nicolas; Rodriguez, Alain L.; Tucker, Charles E. (2004). Organic Reactions. doi:10.1002/0471264180.or058.02. ISBN 0471264180.
- ^ a b The Chemistry of Organozinc Compounds (Patai Series: The Chemistry of Functional Groups), (Eds. Z. Rappoport and I. Marek), John Wiley & Sons: Chichester, UK, 2006, ISBN 0-470-09337-4.
- ^ a b Organozinc reagents – A Practical Approach, (Eds. P. Knochel and P. Jones), Oxford Medical Publications, Oxford, 1999, ISBN 0-19-850121-8.
- ^ Synthetic Methods of Organometallic and Inorganic Chemistry Vol 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury, W.A. Herrmann Ed., ISBN 3-13-103061-5
- ^ E. Frankland, Liebigs Ann. Chem.,1849, 71, 171
- ^ Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2