| |||
| |||
 Oxalic acid dihydrate
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,2-ethanedioic acid
| |||
| Preferred IUPAC name
Oxalic acid[1] | |||
| Systematic IUPAC name
Ethanedioic acid[1] | |||
| Other names
Wood bleach
(Carboxyl)carboxylic acid Carboxylformic acid Dicarboxylic acid Diformic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 385686 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.123 | ||
| EC Number |
| ||
| 2208 | |||
| KEGG | |||
| MeSH | Oxalic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 3261 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H2O4 | |||
| Molar mass | 90.034 g·mol−1 (anhydrous) 126.065 g·mol−1 (dihydrate) | ||
| Appearance | White crystals | ||
| Odor | Odorless | ||
| Density | 1.90 g/cm3 (anhydrous, at 17 °C)[2] 1.653 g/cm3 (dihydrate) | ||
| Melting point | 189 to 191 °C (372 to 376 °F; 462 to 464 K) 101.5 °C (214.7 °F; 374.6 K) dihydrate | ||
| |||
| Solubility | 237 g/L (15 °C) in ethanol 14 g/L (15 °C) in diethyl ether[4] | ||
| Vapor pressure | <0.001 mmHg (20 °C)[5] | ||
| Acidity (pKa) | pKa1 = 1.25 pKa2 = 4.14[6] | ||
| Conjugate base | Hydrogenoxalate | ||
| −60.05·10−6 cm3/mol | |||
| Thermochemistry[7] | |||
Heat capacity (C)
|
91.0 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
109.8 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−829.9 kJ/mol | ||
| Pharmacology | |||
| QP53AG03 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302+H312, H318, H402 | |||
| P264, P270, P273, P280, P301+P312+P330, P302+P352+P312, P305+P351+P338+P310, P362+P364, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 166 °C (331 °F; 439 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published)
|
1000 mg/kg (dog, oral) 1400 mg/kg (rat) 7500 mg/kg (rat, oral)[8] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3[5] | ||
REL (Recommended)
|
TWA 1 mg/m3 ST 2 mg/m3[5] | ||
IDLH (Immediate danger)
|
500 mg/m3[5] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
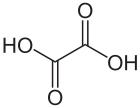
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula HO−C(=O)−C(=O)−OH, also written as (COOH)2 or (CO2H)2 or H2C2O4. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent[9] and its conjugate bases hydrogen oxalate (HC2O−4) and oxalate (C2O2−4) are chelating agents for metal cations. It is used as a cleaning agent, especially for the removal of rust, because it forms a water-soluble ferric iron complex, the ferrioxalate ion. Oxalic acid typically occurs as the dihydrate with the formula H2C2O4·2H2O.
- ^ a b "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Apelblat, Alexander; Manzurola, Emanuel (1987). "Solubility of oxalic, malonic, succinic, adipic, maleic, malic, citric, and tartaric acids in water from 278.15 to 338.15 K". The Journal of Chemical Thermodynamics. 19 (3): 317–320. doi:10.1016/0021-9614(87)90139-X.
- ^ Radiant Agro Chem. "Oxalic Acid MSDS". Archived from the original on 2011-07-15. Retrieved 2012-02-02.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0474". National Institute for Occupational Safety and Health (NIOSH).
- ^ Bjerrum, Jannik; Sillén, Lars Gunnar; Schwarzenbach, Gerold Karl; Anderegg, Giorgio (1958). Stability constants of metal-ion complexes, with solubility products of inorganic substances. London: Chemical Society.
- ^ CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ "Oxalic acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 2005. pp. 17624/28029. doi:10.1002/14356007. ISBN 9783527306732.