 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɒks.kɑːrˈbæz.ɪˌpiːn/ |
| Trade names | Trileptal, Oxtellar XR, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601245 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >95% |
| Metabolism | Liver (cytosolic enzymes and glucuronic acid) |
| Elimination half-life | 1–5 hours (healthy adults) |
| Excretion | Kidney (<1%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.702 |
| Chemical and physical data | |
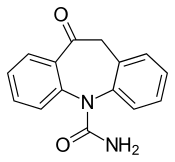
| Formula | C15H12N2O2 |
| Molar mass | 252.273 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxcarbazepine, sold under the brand name Trileptal among others, is a medication used to treat epilepsy.[3][5] For epilepsy it is used for both focal seizures and generalized seizures.[6] It has been used both alone and as add-on therapy in people with bipolar disorder who have had no success with other treatments.[7][5] It is taken by mouth.[3][5]
Common side effects include nausea, vomiting, dizziness, drowsiness, double vision and trouble with walking.[3] Serious side effects may include anaphylaxis, liver problems, pancreatitis, suicide ideation, and an abnormal heart beat.[3][6] While use during pregnancy may harm the baby, use may be less risky than having a seizure.[1][8] Use is not recommended during breastfeeding.[1] In those with an allergy to carbamazepine there is a 25% risk of problems with oxcarbazepine.[3] How it works is not entirely clear.[5]
Oxcarbazepine was patented in 1969 and came into medical use in 1990.[9] It is available as a generic medication.[6] In 2022, it was the 167th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[10][11]
- ^ a b c Cite error: The named reference
Preg2019was invoked but never defined (see the help page). - ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d e f g "Trileptal- oxcarbazepine tablet, film coated Trileptal- oxcarbazepine suspension". DailyMed. Retrieved 9 November 2020.
- ^ "List of nationally authorised medicinal products : Active substance: oxcarbazepine : Procedure no.: PSUSA/00002235/202108" (PDF). Ema.europa.eu. Retrieved 5 June 2022.
- ^ a b c d "Oxcarbazepine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ a b c British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 319–320. ISBN 9780857113382.
- ^ Mazza M, Di Nicola M, Martinotti G, Taranto C, Pozzi G, Conte G, et al. (April 2007). "Oxcarbazepine in bipolar disorder: a critical review of the literature". Expert Opinion on Pharmacotherapy. 8 (5): 649–656. doi:10.1517/14656566.8.5.649. PMID 17376019. S2CID 25068107.
- ^ Athar F, Ehsan M, Farooq M, Lo KB, Cheema HA, Ahmad S, et al. (May 2022). "Adverse fetal and neonatal outcomes following in-utero exposure to oxcarbazepine: A systematic review and meta-analysis". British Journal of Clinical Pharmacology. 88 (8): 3000–09. doi:10.1111/bcp.15413. PMID 35591806.
- ^ Fischer J, Ganellin CR, eds. (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 532. ISBN 9783527607495.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Oxcarbazepine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.