| PAS fold | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
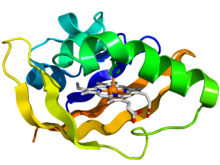 Crystallographic structure of the PAS domain of the bacterial oxygen sensor protein fixL.[1] The protein is depicted as a rainbow colored cartoon (N-terminus = blue, C-terminus = red) while the heme ligand is shown as sticks (carbon = white, nitrogen = blue, oxygen = red, iron = orange). | |||||||||||
| Identifiers | |||||||||||
| Symbol | PAS | ||||||||||
| Pfam | PF00989 | ||||||||||
| Pfam clan | CL0183 | ||||||||||
| ECOD | 223.1.1 | ||||||||||
| InterPro | IPR013767 | ||||||||||
| SMART | PAS | ||||||||||
| PROSITE | PDOC50112 | ||||||||||
| SCOP2 | 2phy / SCOPe / SUPFAM | ||||||||||
| CDD | cd00130 | ||||||||||
| |||||||||||
A Per-Arnt-Sim (PAS) domain is a protein domain found in all kingdoms of life.[2] Generally, the PAS domain acts as a molecular sensor, whereby small molecules and other proteins associate via binding of the PAS domain.[3][4][5] Due to this sensing capability, the PAS domain has been shown as the key structural motif involved in protein-protein interactions of the circadian clock, and it is also a common motif found in signaling proteins, where it functions as a signaling sensor.[6][7]
- ^ PDB: 1y28; Dunham CM, Dioum EM, Tuckerman JR, Gonzalez G, Scott WG, Gilles-Gonzalez MA (July 2003). "A distal arginine in oxygen-sensing heme-PAS domains is essential to ligand binding, signal transduction, and structure". Biochemistry. 42 (25): 7701–8. doi:10.1021/bi0343370. PMID 12820879. S2CID 14090693.
- ^ Henry JT, Crosson S (1 January 2011). "Ligand-binding PAS domains in a genomic, cellular, and structural context". Annual Review of Microbiology. 65: 261–286. doi:10.1146/annurev-micro-121809-151631. PMC 3298442. PMID 21663441.
- ^ Liu YC, Machuca MA, Beckham SA, Gunzburg MJ, Roujeinikova A (October 2015). "Structural basis for amino-acid recognition and transmembrane signalling by tandem Per-Arnt-Sim (tandem PAS) chemoreceptor sensory domains". Acta Crystallographica. Section D, Biological Crystallography. 71 (Pt 10): 2127–2136. Bibcode:2015AcCrD..71.2127L. doi:10.1107/S139900471501384X. PMID 26457436.
- ^ Möglich A, Ayers RA, Moffat K (October 2009). "Structure and signaling mechanism of Per-ARNT-Sim domains". Structure. 17 (10): 1282–1294. doi:10.1016/j.str.2009.08.011. PMC 3092527. PMID 19836329.
- ^ Hennig S, Strauss HM, Vanselow K, Yildiz O, Schulze S, Arens J, et al. (April 2009). "Structural and functional analyses of PAS domain interactions of the clock proteins Drosophila PERIOD and mouse PERIOD2". PLOS Biology. 7 (4): e94. doi:10.1371/journal.pbio.1000094. PMC 2671562. PMID 19402751.
- ^ Ponting CP, Aravind L (November 1997). "PAS: a multifunctional domain family comes to light". Current Biology. 7 (11): R674–R677. doi:10.1016/S0960-9822(06)00352-6. PMID 9382818. S2CID 14105830.
- ^ Hefti MH, Françoijs KJ, de Vries SC, Dixon R, Vervoort J (March 2004). "The PAS fold. A redefinition of the PAS domain based upon structural prediction". European Journal of Biochemistry. 271 (6): 1198–1208. doi:10.1111/j.1432-1033.2004.04023.x. PMID 15009198.