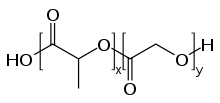
PLGA, PLG, or poly(lactic-co-glycolic) acid (CAS: 26780-50-7 ) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility.[1] PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units (of glycolic or lactic acid) are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.[2]
- ^ Abulateefeh SR (February 2023). "Long-acting injectable PLGA/PLA depots for leuprolide acetate: successful translation from bench to clinic". Drug Delivery and Translational Research. 13 (2): 520–530. doi:10.1007/s13346-022-01228-0. PMID 35976565. S2CID 251622670.
- ^ Astete CE, Sabliov CM (2006). "Synthesis and characterization of PLGA nanoparticles". Journal of Biomaterials Science. Polymer Edition. 17 (3): 247–289. doi:10.1163/156856206775997322. PMID 16689015. S2CID 7607080.