| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
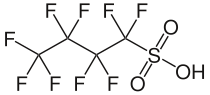
1,1,2,2,3,3,4,4,4-Nonafluorobutane-1-sulfonic acid | |
| Other names
FC-98
Nonaflate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.176 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3094, 3265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4HF9O3S | |
| Molar mass | 300.10 g/mol |
| Boiling point | 210–212 °C (410–414 °F; 483–485 K)[1] |
| Hazards | |
| GHS labelling: | |
  [2] [2]
| |
| Danger | |
| H302, H314 | |
| P280, P305+P351+P338, P310[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perfluorobutanesulfonic acid (PFBS) is a PFAS chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid.[1] Its conjugate base is perfluorobutanesulfonate (also called nonaflate) which functions as the hydrophobe in fluorosurfactants.
Since June 2003, 3M has used PFBS as a replacement for the persistent, toxic, and bioaccumulative compound perfluorooctanesulfonic acid (PFOS) in its Scotchgard stain repellents.[3] 3M markets surfactant with PFBS in two fluorosurfactants.[4]
- ^ a b "Perfluorobutanesulfonic acid". PubChem. NIH. Retrieved 10 August 2021.
- ^ a b Sigma-Aldrich Co., Nonafluorobutane-1-sulfonic acid. Retrieved on 15 January 2018.
- ^ Ullah, Aziz (October 2006). "The Fluorochemical Dilemma: What the PFOS/PFOA fuss is all about" (PDF). Cleaning & Restoration. Retrieved 16 January 2009.
- ^ Renner R (January 2006). "The long and the short of perfluorinated replacements". Environ. Sci. Technol. 40 (1): 12–3. doi:10.1021/es062612a. PMID 16433328.