| |

| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| O8S2−2 | |
| Molar mass | 192.11 g·mol−1 |
| Conjugate acid | Peroxydisulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
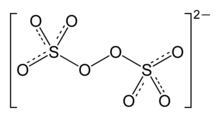
The peroxydisulfate ion, S
2O2−
8, is an oxyanion, the anion of peroxydisulfuric acid. It is commonly referred to as persulfate, but this term also refers to the peroxomonosulfate ion, SO2−
5. It is also called peroxodisulfate.[2] Approximately 500,000 tons of salts containing this anion are produced annually. Important salts include sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), and ammonium persulfate ((NH4)2S2O8). These salts are colourless, water-soluble solids that are strong oxidants.[3]
- ^ Ambiguous—see persulfate
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)