 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Demerol, others |
| Other names | Meperidine (USAN US) |
| Pregnancy category |
|
| Dependence liability | High |
| Addiction liability | High[1] |
| Routes of administration | By mouth, intravenous, intramuscular, intrathecal,[2] subcutaneous, epidural[3] |
| Drug class | Opioid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50–60% (Oral), 80–90% (Oral, in cases of hepatic impairment) |
| Protein binding | 65–75% |
| Metabolism | Liver: CYP2B6, CYP3A4, CYP2C19, Carboxylesterase 1 |
| Metabolites | • Norpethidine • Pethidinic Acid • others |
| Elimination half-life | 2.5–4 hours, 7–11 hours (liver disease) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.299 |
| Chemical and physical data | |
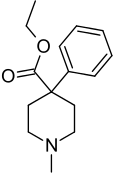
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a fully synthetic opioid pain medication of the phenylpiperidine class.[5][6][7][8][9][10] Synthesized in 1938[11] as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, in Germany.[12] Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (e.g., piminodine, anileridine), the prodines (e.g., alphaprodine, MPPP), bemidones (e.g., ketobemidone), and others more distant, including diphenoxylate and analogues.[13]
Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as a hydrochloride salt in tablets, as a syrup, or by intramuscular, subcutaneous, or intravenous injection. For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1975, 60% of doctors prescribed it for acute pain and 22% for chronic severe pain.[14]
It was patented in 1937 and approved for medical use in 1943.[15] Compared with morphine, pethidine was considered to be safer, carry a lower risk of addiction, and to be superior in treating the pain associated with biliary spasm or renal colic due to its assumed anticholinergic effects.[7] These were later discovered to be inaccurate assumptions, as it carries an equal risk of addiction, possesses no advantageous effects on biliary spasm or renal colic compared to other opioids. Due to the neurotoxicity of its metabolite, norpethidine, it is more toxic than other opioids—especially during long-term use.[7] The norpethidine metabolite was found to have serotonergic effects, so pethidine could, unlike most opioids, increase the risk of triggering serotonin syndrome.[7][8]
- ^ Bonewit-West K, Hunt SA, Applegate E (2012). Today's Medical Assistant: Clinical and Administrative Procedures. Elsevier Health Sciences. p. 571. ISBN 9781455701506. Archived from the original on 10 January 2023. Retrieved 20 August 2019.
- ^ Ngan Kee WD (April 1998). "Intrathecal pethidine: pharmacology and clinical applications". Anaesthesia and Intensive Care. 26 (2): 137–146. doi:10.1177/0310057X9802600202. PMID 9564390.
- ^ Ngan Kee WD (June 1998). "Epidural pethidine: pharmacology and clinical experience". Anaesthesia and Intensive Care. 26 (3): 247–255. doi:10.1177/0310057X9802600303. PMID 9619217.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "Demerol, Pethidine (meperidine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 9 April 2014.
- ^ Shipton E (March 2006). "Should New Zealand continue signing up to the Pethidine Protocol?" (PDF). The New Zealand Medical Journal. 119 (1230): U1875. PMID 16532042. Archived from the original (PDF) on 2014-04-08.
- ^ a b c d Latta KS, Ginsberg B, Barkin RL (January–February 2002). "Meperidine: a critical review". American Journal of Therapeutics. 9 (1): 53–68. doi:10.1097/00045391-200201000-00010. PMID 11782820. S2CID 23410891.
- ^ a b MacPherson RD, Duguid MD (2008). "Strategy to Eliminate Pethidine Use in Hospitals". Journal of Pharmacy Practice and Research. 38 (2): 88–89. doi:10.1002/j.2055-2335.2008.tb00807.x. S2CID 71812645.
- ^ Mather LE, Meffin PJ (September–October 1978). "Clinical pharmacokinetics of pethidine". Clinical Pharmacokinetics. 3 (5): 352–368. doi:10.2165/00003088-197803050-00002. PMID 359212. S2CID 35402662.
- ^ Cite error: The named reference
AMHwas invoked but never defined (see the help page). - ^ US 2167351 Piperidine compounds and a process of preparing them
- ^ Michaelis M, Schölkens B, Rudolphi K (April 2007). "An anthology from Naunyn-Schmiedeberg's archives of pharmacology". Naunyn-Schmiedeberg's Archives of Pharmacology. 375 (2): 81–84. doi:10.1007/s00210-007-0136-z. PMID 17310263. S2CID 27774155.
- ^ Reynolds AK, Randall LO (1957). Morphine and Allied Drugs. Toronto/London: University of Toronto Press/Oxford University Press. pp. 273–319.
- ^ Kaiko RF, Foley KM, Grabinski PY, Heidrich G, Rogers AG, Inturrisi CE, Reidenberg MM (February 1983). "Central nervous system excitatory effects of meperidine in cancer patients". Annals of Neurology. 13 (2): 180–185. doi:10.1002/ana.410130213. PMID 6187275. S2CID 44353966.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 52X. ISBN 9783527607495.