This article may be confusing or unclear to readers. In particular, the lead states that weight loss stops after a few weeks, but "Medical use" states that it continues to occur "through the ninth month", so which is it?. (May 2021) |
 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Ionamin, Suprenza, others | ||
| Other names | α-methyl-amphetamine α,α-dimethylphenethylamine | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a682187 | ||
| Pregnancy category |
| ||
| Dependence liability | Physical: not typical Psychological: Moderate[1] | ||
| Addiction liability | Low[2] | ||
| Routes of administration | By mouth | ||
| Drug class | Appetite suppressant[3] | ||
| ATC code | |||
| Legal status | |||
| Legal status |
| ||
| Pharmacokinetic data | |||
| Bioavailability | High (almost complete)[5] | ||
| Protein binding | Approximately 96.3% | ||
| Metabolism | Liver[5] | ||
| Elimination half-life | 25 hours, urinary pH-dependent[5] | ||
| Excretion | Kidney (62–85% unchanged)[5] | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.112 | ||
| Chemical and physical data | |||
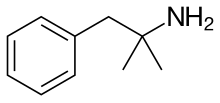
| Formula | C10H15N | ||
| Molar mass | 149.237 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| (verify) | |||
Phentermine (phenyl-tertiary-butyl amine), sold under the brand name Ionamin among others, is a medication used together with diet and exercise to treat obesity.[3] It is taken by mouth for up to a few weeks at a time, after which the benefits subside.[3] It is also available as the combination phentermine/topiramate.[6]
Common side effects include a fast heart beat, high blood pressure, trouble sleeping, dizziness, and restlessness.[3] Serious side effects may include abuse, but do not include pulmonary hypertension or valvular heart disease, as the latter were caused by the fenfluramine component of the fen-phen drug combination.[3] Use is not recommended during pregnancy or breastfeeding,[7] or with SSRIs or MAO inhibitors.[3] It works mainly as an appetite suppressant, likely as a result of being a CNS stimulant.[3] Chemically, phentermine is a substituted amphetamine.[8]
Phentermine was approved for medical use in the United States in 1959.[3] It is available as a generic medication.[3] In 2022, it was the 149th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[9][10] Phentermine was withdrawn from the market in the United Kingdom in 2000, while the combination medication fen-phen, of which it was a part, was withdrawn from the market in 1997 due to side effects[11] of fenfluramine which caused increased levels of circulating serotonin which stimulated serotonin receptors on heart valves and thus causing valve insufficiency and leading to primary pulmonary hypertension (PPH). According to the NIH (National Institutes of Health) there is no evidence that phentermine causes PPH.[citation needed]
- ^ Tarascon Pocket Pharmacopoeia 2017 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2016. p. 7. ISBN 9781284118971.
- ^ Sadock BJ, Sadock VA (2010). Kaplan and Sadock's Pocket Handbook of Clinical Psychiatry. Lippincott Williams & Wilkins. p. 435. ISBN 9781605472645.
- ^ a b c d e f g h i "Phentermine Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 13 April 2019.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b c d Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ "Phentermine and topiramate Uses, Side Effects & Warnings". Drugs.com. Retrieved 13 April 2019.
- ^ "Phentermine Use During Pregnancy". Drugs.com. Retrieved 13 April 2019.
- ^ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (July 2012). "Biosynthesis of amphetamine analogs in plants". Trends in Plant Science. 17 (7): 404–412. Bibcode:2012TPS....17..404H. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Phentermine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Bagchi D, Preuss HG (2012). Obesity: Epidemiology, Pathophysiology, and Prevention (Second ed.). CRC Press. p. 314. ISBN 9781439854259.

