| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenylboronic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 970972 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.456 |
| EC Number |
|
| 3328 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H7BO2 | |
| Molar mass | 121.93 g/mol |
| Appearance | white to yellow powder |
| Odor | odorless |
| Melting point | 216 °C (421 °F; 489 K) |
| 10 g/L (20 °C)[1] | |
| Solubility | soluble in diethyl ether, ethanol |
| Acidity (pKa) | 8.83 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-719.6 kJ/mol |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H302 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
740 mg/ml (rat, oral) |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
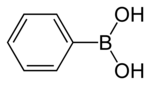
Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is a white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis.
- ^ "Phenylboronic acid | 98-80-6".
- ^ "Phenylboronic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 27 December 2021.