This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Phenotropil, Fenotropil, Phenotropyl, Fenotropyl, Carphedon, Actitropil |
| Other names | Fonturacetam; Phenotropil; Fenotropil; 4-Phenylpiracetam; PP[1] |
| Pregnancy category |
|
| Routes of administration | Oral (tablets)[2][3] |
| Drug class | Atypical dopamine reuptake inhibitor[4] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100%[2][3] |
| Metabolism | Not metabolized[3] |
| Onset of action | <1 hour[3][2] |
| Elimination half-life | 3–5 hours[2][3] |
| Excretion | Urine: ~40%[3] Bile, sweat: ~60%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.214.874 |
| Chemical and physical data | |
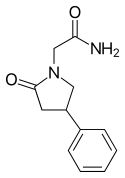
| Formula | C12H14N2O2 |
| Molar mass | 218.256 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Boiling point | 486.4 °C (907.5 °F) |
| |
| |
| (verify) | |
Phenylpiracetam, also known as fonturacetam (INN) and sold under the brand names Phenotropil, Actitropil, and Carphedon among others, is a stimulant and nootropic medication used in Russia and certain other Eastern European countries in the treatment of cerebrovascular deficiency, depression, apathy, and attention, and memory problems, among other indications.[2][4][1][3] It is also used in Russian cosmonauts to improve physical, mental, and cognitive abilities.[2][1] The drug is taken by mouth.[2]
Side effects of phenylpiracetam include sleep disturbances among others.[2] The mechanism of action of phenylpiracetam was originally unknown.[2][4][5] However, it was discovered that (R)-phenylpiracetam is a selective atypical dopamine reuptake inhibitor in 2014.[4][6] In addition, phenylpiracetam interacts with certain nicotinic acetylcholine receptors.[5] Chemically, phenylpiracetam is a racetam and phenethylamine and is structurally related to piracetam.[2][7]
Phenylpiracetam was first described by 1983.[8] It was approved for medical use in Russia in 2003.[2] Development of (R)-phenylpiracetam (code name MRZ-9547) in the West as a potential treatment for fatigue related to Parkinson's disease began by 2014.[9][6]
- ^ a b c Gromova OA, Torshin IY (2024). "Farmakologičeskie èffekty fonturacetama (Aktitropil) i perspektivy ego kliničeskogo primenenija" [Pharmacological effects of fonturacetam (Actitropil) and prospects for its clinical use]. Zh Nevrol Psikhiatr Im S S Korsakova [S.S. Korsakov Journal of Neurology and Psychiatry] (in Russian). 124 (8): 21–31. doi:10.17116/jnevro202412408121. PMID 39269293.
- ^ a b c d e f g h i j k Malykh AG, Sadaie MR (February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs. 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- ^ a b c d e f g h https://russianmeds.com/pdf/fonturacetam.pdf
- ^ a b c d Veinberg G, Vavers E, Orlova N, Kuznecovs J, Domracheva I, Vorona M, et al. (2015). "Stereochemistry of phenylpiracetam and its methyl derivative: improvement of the pharmacological profile". Chemistry of Heterocyclic Compounds. 51 (7): 601–606. doi:10.1007/s10593-015-1747-9. ISSN 0009-3122.
Phenylpiracetam was originally designed as a nootropic drug for the sustenance and improvement of the physical condition and cognition abilities of Soviet space crews.2 Later, especially during the last decade, phenylpiracetam was introduced into general clinical practice in Russia and in some Eastern European countries. The possible target receptors and mechanisms for the acute activity of this drug remained unclear, until very recently it was found that (R)-phenylpiracetam (5) (MRZ-9547) is a selective dopamine transporter inhibitor that moderately stimulates striatal dopamine release.19
- ^ a b Voronina TA (2023). "Cognitive Impairment and Nootropic Drugs: Mechanism of Action and Spectrum of Effects". Neurochemical Journal. 17 (2). Pleiades Publishing Ltd: 180–188. doi:10.1134/s1819712423020198. ISSN 1819-7124.
Phenylpiracetam, a phenyl analogue of piracetam (trade names: phenotropil, carphedon, and phenylpiracetam), was developed at the Institute of Biomedical Problems as a new generation psychostimulant that can increase the mental and physical performance of astronauts at various stages of space flights. It was experimentally established that phenylpiracetam improves learning and memory, has an antiamnesic effect, activates operant behavior, has anxiolytic, antiasthenic, and anticonvulsant effects, weakens the sedative effect of benzodiazepines, increases resistance to cold, and improves sleep [29–31]. In a model of cerebral ischemia, phenylpiracetam improves cognitive functions, reduces manifestations of neurological deficit, and is superior in effectiveness to piracetam [32, 33]. It has been shown that phenylpiracetam does not bind to GABA-A, GABA-B and dopamine receptors, or 5-HT2 serotonin receptor, but is a synaptic transmission modulator and binds to α4β2 nicotinic acetylcholine receptors in the cerebral cortex (IC50 = 5.86 μm) [34, 35].
- ^ a b Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W (December 2014). "The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats". The International Journal of Neuropsychopharmacology. 17 (12): 2045–2056. doi:10.1017/S1461145714000996. PMID 24964269.
Here, we tested the effects of MRZ-9547 [...], and its l-enantiomer MRZ-9546 on effort-related decision making in rats. The racemic form of these compounds referred to as phenotropil has been shown to stimulate motor activity in rats (Zvejniece et al., 2011) and enhance physical capacity and cognition in humans (Malykh and Sadaie, 2010). [...] MRZ-9547 turned out to be a DAT inhibitor as shown by displacement of binding of [125I] RTI-55 (IC50 = 4.82 ± 0.05 μM, n=3) to human recombinant DAT expressed in CHO-K1 cells and inhibition of DA uptake (IC50 = 14.5 ± 1.6 μM, n=2) in functional assays in the same cells. It inhibited norepinephrine transporter (NET) with an IC50 of 182 μM (one experiment in duplicate). The potencies for the l-enantiomer MRZ-9546 were as follows: DAT binding (Ki = 34.8 ± 14.8 μM, n=3), DAT function (IC50 = 65.5 ± 8.3 μM, n=2) and NET function (IC50 = 667 μM, one experiment performed in duplicate).
- ^ Gouliaev AH, Senning A (May 1994). "Piracetam and other structurally related nootropics". Brain Res Brain Res Rev. 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686.
As mentioned above, no commonly accepted mechanism for the racetam nootropics has yet been established, They do not seem to act on any well characterised receptor site with the exception of nefiracetam which has high affinity for GABA, receptors (see Table 1). [...] Receptor: GABAA. Receptor ligand: [3H]muscimolA. Compound: nefiracetam. IC50: 8.5 nMB. Refs.: 222. [...] From Table 1 it is, however, evident that the piracetam-like nootropics do not exhibit high affinity for any of the receptor types tested so far (except for nefiracetam, which shows some activity at GABAA receptors)
- ^ Cite error: The named reference
BobkovMorozovGlozman1983was invoked but never defined (see the help page). - ^ Cite error: The named reference
AdisInsight-MRZ-9547was invoked but never defined (see the help page).
