| phosphoenolpyruvate mutase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 5.4.2.9 | ||||||||
| CAS no. | 115756-49-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
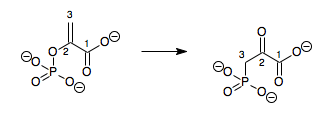
In enzymology, a phosphoenolpyruvate mutase (EC 5.4.2.9) is an enzyme that catalyzes the chemical reaction
- phosphoenolpyruvate 3-phosphonopyruvate
Hence, this enzyme has one substrate, phosphoenolpyruvate (PEP), and one product, 3-phosphonopyruvate (PPR), which are structural isomers.
This enzyme belongs to the family of isomerases, specifically the phosphotransferases (phosphomutases), which transfer phosphate groups within a molecule. The systematic name of this enzyme class is phosphoenolpyruvate 2,3-phosphonomutase. Other names in common use include phosphoenolpyruvate-phosphonopyruvate phosphomutase, PEP phosphomutase, phosphoenolpyruvate phosphomutase, PEPPM, and PEP phosphomutase. This enzyme participates in aminophosphonate metabolism.
Phosphoenolpyruvate mutase was discovered in 1988.[1][2]
- ^ Bowman E, McQueney M, Barry RJ, Dunaway-Mariano D (1988). "Catalysis and thermodynamics of the phosphoenolpyruvate phosphonopyruvate rearrangement - entry into the phosphonate class of naturally-occurring organo-phosphorus compounds". J. Am. Chem. Soc. 110 (16): 5575–5576. doi:10.1021/ja00224a054.
- ^ Seidel HM, Freeman S, Seto H, Knowles JR (1988). "Phosphonate biosynthesis: isolation of the enzyme responsible for the formation of a carbon-phosphorus bond". Nature. 335 (6189): 457–458. Bibcode:1988Natur.335..457S. doi:10.1038/335457a0. PMID 3138545. S2CID 4310660.