| |
| Names | |
|---|---|
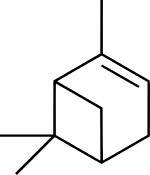
| IUPAC names
(1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene
(1S,5S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.170 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Appearance | Liquid |
| Density | 0,86 g·cm−3 (alpha, 15 °C)[1][2] |
| Melting point | −62 to −55 °C (−80 to −67 °F; 211 to 218 K) (alpha)[1] |
| Boiling point | 155 to 156 °C (311 to 313 °F; 428 to 429 K) (alpha)[1] |
| Practically insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pinene is a collection of unsaturated bicyclic monoterpenes. Two geometric isomers of pinene are found in nature, α-pinene and β-pinene. Both are chiral. As the name suggests, pinenes are found in pines. Specifically, pinene is the major component of the liquid extracts of conifers.[3] Pinenes are also found in many non-coniferous plants such as camphorweed (Heterotheca)[4] and big sagebrush (Artemisia tridentata).
- ^ a b c Record of alpha-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 07-January-2016.
- ^ Record of beta-Pinen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 07-January-2016.
- ^ Gscheidmeier, Manfred; Fleig, Helmut (2000). "Turpentines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_267. ISBN 3527306730.
- ^ Lincoln DE, Lawrence BM (1984). "The Volatile Constituents of Camphorweed, Heterotheca subaxillaris". Phytochemistry. 23 (4): 933–934. Bibcode:1984PChem..23..933L. doi:10.1016/S0031-9422(00)85073-6.