| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
plutonium(VI) fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| PuF 6 | |||
| Appearance | Dark red, opaque crystals | ||
| Density | 5.08 g·cm−3 | ||
| Melting point | 52 °C (126 °F; 325 K) | ||
| Boiling point | 62 °C (144 °F; 335 K) | ||
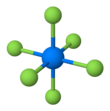
| Structure | |||
| Orthorhombic, oP28 | |||
| Pnma, No. 62 | |||
| octahedral (Oh) | |||
| 0 D | |||
| Related compounds | |||
Related fluoroplutoniums
|
Plutonium trifluoride | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Plutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This isotope of plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.