 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /poʊˈnætɪnɪb/ poh-NAT-i-nib |
| Trade names | Iclusig |
| Other names | AP24534 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613029 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | >99% (in vitro) |
| Metabolism | Liver (CYP3A4, 2C8, 2D6, 3A5) |
| Elimination half-life | 12–66 hours |
| Excretion | Feces (87%), urine (5%)[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
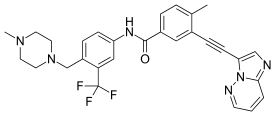
| Formula | C29H27F3N6O |
| Molar mass | 532.571 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ponatinib, sold under the brand name Iclusig, is a medication used for the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia.[4] It was developed by Ariad Pharmaceuticals. It is a multi-targeted tyrosine-kinase inhibitor.[5] Some forms of chronic myeloid leukemia, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.[6]
The United States Food and Drug Administration (FDA) approved the medication as a candidate in December 2012, but temporarily suspended sales in October 2013, because of "the risk of life-threatening blood clots and severe narrowing of blood vessels".[7][8] The suspension was partially lifted on in December 2013, with ponatinib being issued revised prescribing information, a new "Black Box Warning" and a "Risk Evaluation and Mitigation Strategy" in place to better evaluate the risks and benefits of using the drug.[citation needed]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2014". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. 7 July 2016. Retrieved 1 April 2024.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ a b c "Iclusig- ponatinib hydrochloride tablet, film coated". DailyMed. 10 November 2022. Archived from the original on 6 October 2022. Retrieved 11 April 2023.
- ^ Huang WS, Metcalf CA, Sundaramoorthi R, Wang Y, Zou D, Thomas RM, et al. (June 2010). "Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant". Journal of Medicinal Chemistry. 53 (12): 4701–4719. doi:10.1021/jm100395q. PMID 20513156.
- ^ O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. (November 2009). "AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance". Cancer Cell. 16 (5): 401–412. doi:10.1016/j.ccr.2009.09.028. PMC 2804470. PMID 19878872.
- ^ "FDA asks manufacturer of the leukemia drug Iclusig (ponatinib) to suspend marketing and sales". U.S. Food and Drug Administration. 31 October 2013. Archived from the original on 2 November 2017. Retrieved 16 December 2019.
- ^ Grady D (31 October 2013). "Serious Danger of Blood Clots Halts Sale of Leukemia Drug". The New York Times. Archived from the original on 18 September 2021. Retrieved 28 February 2017.