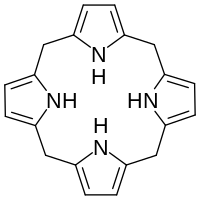
In biochemistry, a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle of four pyrrole rings connected by four methylene bridges.[1] They can be viewed as derived from the parent compound hexahydroporphine by the substitution of various functional groups for hydrogen atoms in the outermost (20-carbon) ring.
Porphyrinogens are intermediates in the biosynthesis of porphyrins, cofactors with a porphine core which are found in many enzymes and proteins including myoglobin, hemoglobin, cytochromes, and chlorophylls.[2]
Porphyrins differ from porphyrinogens by having the four pyrrole rings linked by methine bridges =CH− instead of methylene bridges −CH2−, and by lacking the hydrogen atom in two of the four amine −NH− groups, turning them into imines =N−. In the biosynthesis of porphyrins, the parent porphyrinogen is dehydrogenated by protoporphyrinogen oxidase.
Because of their limited delocalization, porphyrinogens are colorless. Loss of all four central hydrogen atoms in the core yields a tetravalent anion that can act as a ligand to metal cations, creating a coordination compound.[3] Subsequent biosynthetic intermediates en route to porphyrins are deeply colored and often phytotoxic.
- ^ porphyrinogens - IUPAC Gold Book
- ^ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- ^ Cite error: The named reference
sesslerwas invoked but never defined (see the help page).