| |
| Names | |
|---|---|
| IUPAC name
Dipotassium heptafluorotantalate
| |
| Systematic IUPAC name
Dipotassium heptafluorotantalum(2-) | |
| Other names
Potassium heptafluorotantalate(V)
Potassium fluorotantalate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.245 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
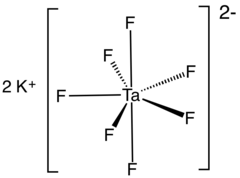
| K2[TaF7] | |
| Molar mass | 392.13 g/mol |
| Appearance | white solid |
| Density | 4.56 g/mL at 25 °C |
| Melting point | 630 to 820 °C (1,166 to 1,508 °F; 903 to 1,093 K) |
0.5 g/100 mL (15 °C)[1]
| |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H301, H315, H319, H331, H335 | |
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P311, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
110 mg/kg (Oral: rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium heptafluorotantalate is an inorganic compound with the formula K2[TaF7]. It is the potassium salt of the heptafluorotantalate anion [TaF7]2−. This white, water-soluble solid is an intermediate in the purification of tantalum from its ores and is the precursor to the metal.[2]