
| |
| Names | |
|---|---|
| IUPAC name
potassium hexafluoronickelate(IV)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.153.655 |
| EC Number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| K2NiF6 | |
| Molar mass | 250.880 |
| Hazards[1] | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H312, H317, H331, H350 | |
| P201, P261, P280, P304+P340, P405, P501 | |
| Safety data sheet (SDS) | External SDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium hexafluoronickelate(IV) is an inorganic compound with the chemical formula K
2NiF
6. It can be produced through the reaction of potassium fluoride, nickel dichloride, and fluorine.
It reacts violently with water, releasing oxygen. It dissolves in anhydrous hydrogen fluoride to produce a light-red solution. Potassium hexafluoronickelate(IV) decomposes at 350 °C, forming potassium hexafluoronickelate(III), nickel(II) fluoride, and fluorine:[2][better source needed][3]
Potassium hexafluoronickelate is a strong oxidant. It can turn chlorine pentafluoride and bromine pentafluoride into ClF+
6 and BrF+
6, respectively:[4]
- ( X = Cl or Br , -60 °C , aHF = anhydrous hydrogen fluoride).
Potassium hexafluoronickelate decomposes at high temperatures to release fluorine gas; like terbium(IV) fluoride, the emitted fluorine is primarily monatomic rather than the typical diatomic.[5]
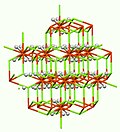
It adopts the structure seen for K2PtCl6 and Mg2FeH6.[6]
- ^ "Potassium Hexafluoronickelate(IV)". American Elements. Retrieved December 19, 2018.
- ^ (in Chinese)张青莲. 《无机化学丛书》第九卷:锰分族、铁系、铂系. 北京: 科学出版社. pp. P333. ISBN 7-03-002238-6.
- ^ Stein, Lawrence; Neil, John M.; Alms, Gregory R. (November 1969). "Properties of potassium hexafluoronickelates(III) and -(IV). Absorption spectra of nickel(III) and -(IV) in hydrogen fluoride solutions". Inorganic Chemistry. 8 (11): 2472–2476. doi:10.1021/ic50081a045. ISSN 0020-1669.
- ^ Schroer, Thorsten; Christe, Karl O. (2001). "Novel Synthesis of ClF6+ and BrF6+ Salts". Inorganic Chemistry. 40 (10): 2415–9. doi:10.1021/ic001024. PMID 11327921.
- ^ Rau, J. V.; Chilingarov, N. S.; Leskiv, M. S.; Sukhoverkhov, V. F.; Rossi Albertini, V.; Sidorov, L. N. (August 2001). "Transition and rare earth metal fluorides as thermal sources of atomic and molecular fluorine". Le Journal de Physique IV. 11 (PR3): Pr3–109–Pr3-113. doi:10.1051/jp4:2001314.
- ^ Taylor, J. C. "A comparison of profile decomposition and Rietveld methods for structurtal refinement with powder diffraction data" Zeitschrift für Kristallographie 1987, volume 181, p151-160.

