| |
| Names | |
|---|---|
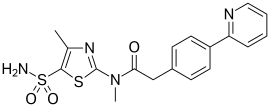
| Systematic IUPAC name
N-Methyl-N-(4-methyl-5-sulfamoyl-1,3-thiazol-2-yl)-2-[4-(pyridin-2-yl)phenyl]acetamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H18N4O3S2 | |
| Molar mass | 402.49 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pritelivir (development codes AIC316 or BAY 57-1293) is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV). This is particularly important in immune compromised patients. It is currently in Phase III clinical development by the German biopharmaceutical company AiCuris Anti-infective Cures AG. US FDA granted fast track designation for pritelivir in 2017 and breakthrough therapy designation 2020.