 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paludrine, others |
| Other names | chlorguanide, chloroguanide[1] |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 75% |
| Metabolism | By liver (CYP2C19) |
| Metabolites | cycloguanil and 4-chlorophenylbiguanide |
| Elimination half-life | 12–21 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.196 |
| Chemical and physical data | |
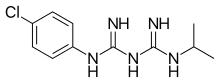
| Formula | C11H16ClN5 |
| Molar mass | 253.73 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 129 °C (264 °F) |
| |
| |
| (verify) | |
Proguanil, also known as chlorguanide and chloroguanide, is a medication used to treat and prevent malaria.[3][4] It is often used together with chloroquine or atovaquone.[4][3] When used with chloroquine the combination will treat mild chloroquine resistant malaria.[3] It is taken by mouth.[4]
Side effects include diarrhea, constipation, skin rashes, hair loss, and itchiness.[3] Because malaria tends to be more severe in pregnancy, the benefit typically outweighs the risk.[3] If used during pregnancy it should be taken with folate.[3] It is likely safe for use during breastfeeding.[3] Proguanil is converted by the liver to its active metabolite, cycloguanil.[4]
It is on the World Health Organization's List of Essential Medicines.[5] In the United States and Canada it is only available in combination as atovaquone/proguanil.[6]
- ^ Mehlhorn H (2008). "Proguanil". Encyclopedia of Parasitology: A-M. Springer Science & Business Media. p. 388. ISBN 9783540489948. Archived from the original on 20 December 2016.
- ^ "Malarone (atovaquone/proguanil) Tablets, Pediatric Tablets. Full Prescribing Information" (PDF). GlaxoSmithKline. Research Triangle Park, NC 27709. Archived (PDF) from the original on 20 September 2016. Retrieved 14 July 2016.
- ^ a b c d e f g World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 199, 203. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d "Atovaquone and Proguanil Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Proguanil". www.medscape.com. Medscape. Archived from the original on 9 November 2016. Retrieved 8 November 2016.