 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Phenergan, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682284 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intravenous, intramuscular, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 88% absorbed but after first-pass metabolism reduced to 25% absolute bioavailability[2] |
| Protein binding | 93% |
| Metabolism | Liver glucuronidation and sulfoxidation |
| Elimination half-life | 10–19 hours[2][3] |
| Excretion | Kidney and Bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.445 |
| Chemical and physical data | |
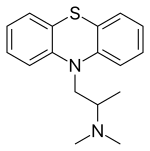
| Formula | C17H20N2S |
| Molar mass | 284.42 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Promethazine, sold under the brand name Phenergan among others, is a first-generation antihistamine, sedative, and antiemetic used to treat allergies, insomnia, and nausea. It may also help with some symptoms associated with the common cold[4] and may also be used for sedating people who are agitated or anxious, an effect that has led to some recreational use (especially with codeine).[5][6][7] Promethazine is taken by mouth (oral), as a rectal suppository, or by injection into a muscle (IM).[4]
Common side effects of promethazine include confusion and sleepiness;[4] consumption of alcohol or other sedatives can make these symptoms worse.[4] It is unclear if use of promethazine during pregnancy or breastfeeding is safe for the fetus.[4][6] Use of promethazine is not recommended in those less than two years old, due to potentially negative effects on breathing.[4] Use of promethazine by injection into a vein is not recommended, due to potential skin damage.[4] Promethazine is in the phenothiazine family of medications.[4] It is also a strong anticholinergic, which produces its sedative effects. This also means high or toxic doses can act as a deliriant.[8]
Promethazine was made in the 1940s by a team of scientists from Rhône-Poulenc laboratories.[9] It was approved for medical use in the United States in 1951.[4] It is a generic medication and is available under many brand names globally.[1] In 2022, it was the 198th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[10][11] In 2022, the combination with dextromethorphan was the 260th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[10][12]
- ^ a b "Promethazine international brands". Drugs.com. Retrieved 17 July 2017.
- ^ a b Cite error: The named reference
pmid10965395was invoked but never defined (see the help page). - ^ Paton DM, Webster DR (1985). "Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines)". Clinical Pharmacokinetics. 10 (6): 477–97. doi:10.2165/00003088-198510060-00002. PMID 2866055. S2CID 33541001.
- ^ a b c d e f g h i "Promethazine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 24 October 2018.
- ^ Cite error: The named reference
leanwas invoked but never defined (see the help page). - ^ a b British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. 276. ISBN 978-0-85711-298-9.
- ^ Malamed SF (2009). Sedation: A Guide to Patient Management. Elsevier Health Sciences. p. 113. ISBN 978-0-323-07596-1.
- ^ Page CB, Duffull SB, Whyte IM, Isbister GK (February 2009). "Promethazine overdose: clinical effects, predicting delirium and the effect of charcoal". QJM. 102 (2): 123–131. doi:10.1093/qjmed/hcn153. PMID 19042969. S2CID 17677540.
- ^ Li JJ (2006). Laughing Gas, Viagra, and Lipitor: The Human Stories behind the Drugs We Use. United Kingdom: Oxford University Press. p. 146. ISBN 978-0-19-988528-2. Retrieved 9 July 2016.
- ^ a b "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Promethazine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Dextromethorphan; Promethazine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.