 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diprivan, others[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: Very High Psychological: no data |
| Addiction liability | Moderate[2] |
| Routes of administration | Intravenous |
| Drug class | GABA receptor agonist; sedative; hypnotic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 95–99% |
| Metabolism | Liver glucuronidation |
| Onset of action | 15–30 seconds[5] |
| Elimination half-life | 1.5–31 hours[5] |
| Duration of action | ~5–10 minutes[5] |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.551 |
| Chemical and physical data | |
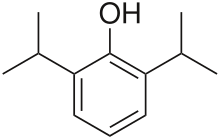
| Formula | C12H18O |
| Molar mass | 178.275 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | ΔGsolvH2O = -4.39kcal/mol[6] |
| |
| |
| (verify) | |
Propofol[7] is the active component of an intravenous anesthetic formulation used for induction and maintenance of general anesthesia. It is chemically termed 2,6-diisopropylphenol. The formulation was approved under the brand name Diprivan. Numerous generic versions have since been released. Intravenous administration is used to induce unconsciousness after which anesthesia may be maintained using a combination of medications. It is manufactured as part of a sterile injectable emulsion formulation using soybean oil and lecithin, giving it a white milky coloration.[8]
Recovery from propofol-induced anesthesia is generally rapid and associated with less frequent side effects[9][10] (e.g. drowsiness, nausea, vomiting) compared to other anesthetic agents. Propofol may be used prior to diagnostic procedures requiring anesthesia, in the management of refractory status epilepticus, and for induction and/or maintenance of anesthesia prior to and during surgeries. It may be administered as a bolus or an infusion, or some combination of the two.
First synthesized in 1973, by John B. Glen, a British veterinary anesthesiologist working for Imperial Chemical Industries (ICI, later AstraZeneca),[11] in 1986 propofol was introduced for therapeutic use as a lipid emulsion in the United Kingdom and New Zealand. Propofol (Diprivan) received FDA approval in October 1989. It is on the World Health Organization's List of Essential Medicines.[12]
- ^ "Propofol". Drugs.com. Retrieved 2 January 2019.
- ^ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". The American Journal of Drug and Alcohol Abuse. 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822. S2CID 19157711.
Propofol is a general anesthetic, however its abuse for recreational purpose has been documented (120). Using control drugs implicated in both ΔFosB induction and addiction (ethanol and nicotine), similar ΔFosB expression was apparent when propofol was given to rats. Moreover, this cascade was shown to act via the dopamine D1 receptor in the NAc, suggesting that propofol has abuse potential (119)
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Diprivan- propofol injection, emulsion". DailyMed. Retrieved 17 April 2021.
- ^ a b c "Propofol". The American Society of Health-System Pharmacists. Archived from the original on 9 October 2016. Retrieved 21 January 2017.
- ^ Arcario MJ, Mayne CG, Tajkhorshid E (October 2014). "Atomistic models of general anesthetics for use in in silico biological studies". The Journal of Physical Chemistry B. 118 (42). American Chemical Society (ACS): 12075–12086. doi:10.1021/jp502716m. PMC 4207551. PMID 25303275.
- ^ "Propofol". PubChem. U.S. National Library of Medicine. Retrieved 25 October 2023.
- ^ "Making Stable, Sterile Propofol". www.microfluidics-mpt.com. Retrieved 25 October 2023.
- ^ "Propofol". go.drugbank.com. Retrieved 25 October 2023.
- ^ Glen JB (25 September 2018). "The Discovery and Development of Propofol Anesthesia: The 2018 Lasker-DeBakey Clinical Medical Research Award". JAMA. 320 (12): 1235–1236. doi:10.1001/jama.2018.12756. ISSN 0098-7484. PMID 30208399.
- ^ Glen JB (July 2019). "Try, try, and try again: personal reflections on the development of propofol". British Journal of Anaesthesia. 123 (1): 3–9. doi:10.1016/j.bja.2019.02.031. PMID 30982566. S2CID 115198733.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.