 | |
| Clinical data | |
|---|---|
| Trade names | Resolor, Resotran, Motegrity |
| Other names | R-093877, R-108512 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
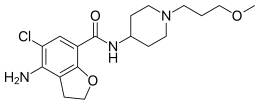
| Formula | C18H26ClN3O3 |
| Molar mass | 367.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Prucalopride, sold under brand names Resolor and Motegrity among others, is a medication acting as a selective, high affinity 5-HT4 receptor agonist[4] which targets the impaired motility associated with chronic constipation, thus normalizing bowel movements.[5][6][7][8][9][10] Prucalopride was approved for medical use in the European Union in 2009,[3] in Canada in 2011,[11] in Israel in 2014,[12] and in the United States in December 2018.[13] The drug has also been tested for the treatment of chronic intestinal pseudo-obstruction.[14][15]
- ^ "Prucalopride succinate (MedTAS Pty Ltd)". Therapeutic Goods Administration (TGA). 7 October 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ "Motegrity- prucalopride tablet, film coated". DailyMed. 28 October 2022. Retrieved 13 September 2024.
- ^ a b "Resolor EPAR". European Medicines Agency (EMA). 17 November 2009. Archived from the original on 31 January 2010.
- ^ Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, et al. (June 2001). "The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound". European Journal of Pharmacology. 423 (1): 71–83. doi:10.1016/S0014-2999(01)01087-1. PMID 11438309.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Clinical trial number NCT00793247 for "Efficacy Study of Prucalopride to Treat Chronic Intestinal Pseudo-Obstruction (CIP)" at ClinicalTrials.gov
- ^ Emmanuel AV, Kamm MA, Roy AJ, Kerstens R, Vandeplassche L (January 2012). "Randomised clinical trial: the efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction--a double-blind, placebo-controlled, cross-over, multiple n = 1 study". Alimentary Pharmacology & Therapeutics. 35 (1): 48–55. doi:10.1111/j.1365-2036.2011.04907.x. PMC 3298655. PMID 22061077.
- ^ Smart CJ, Ramesh AN (August 2012). "The successful treatment of acute refractory pseudo-obstruction with prucalopride". Colorectal Disease. 14 (8): e508. doi:10.1111/j.1463-1318.2011.02929.x. PMID 22212130. S2CID 29060148.
- ^ Bouras EP, Camilleri M, Burton DD, McKinzie S (May 1999). "Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans". Gut. 44 (5): 682–6. doi:10.1136/gut.44.5.682. PMC 1727485. PMID 10205205.
- ^ Bouras EP, Camilleri M, Burton DD, Thomforde G, McKinzie S, Zinsmeister AR (February 2001). "Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder". Gastroenterology. 120 (2): 354–60. doi:10.1053/gast.2001.21166. PMID 11159875.
- ^ Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L (March 2009). "Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives". Gut. 58 (3): 357–65. doi:10.1136/gut.2008.162404. PMID 18987031. S2CID 206948212.
- ^ "Health Canada, Notice of Decision for Resotran". hc-sc.gc.ca. Archived from the original on 18 March 2017. Retrieved 1 May 2018.
- ^ "Digestive Remedies in Israel". www.euromonitor.com. Archived from the original on 13 March 2018. Retrieved 1 May 2018.
- ^ "Drug Approval Package: Motegrity (prucalopride)". U.S. Food and Drug Administration (FDA). 28 December 2018. Archived from the original on 8 October 2020. Retrieved 3 October 2020.
- ^ Briejer MR, Prins NH, Schuurkes JA (October 2001). "Effects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogs". Neurogastroenterology and Motility. 13 (5): 465–72. doi:10.1046/j.1365-2982.2001.00280.x. PMID 11696108. S2CID 13610558.
- ^ Oustamanolakis P, Tack J (February 2012). "Prucalopride for chronic intestinal pseudo-obstruction". Alimentary Pharmacology & Therapeutics. 35 (3): 398–9. doi:10.1111/j.1365-2036.2011.04947.x. PMID 22221087. S2CID 5526239.