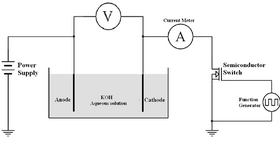
Pulse electrolysis is an alternate electrolysis method that utilises a pulsed direct current to initiate non-spontaneous chemical reactions.[1][2][3] Also known as pulsed direct current (PDC) electrolysis, the increased number of variables that it introduces to the electrolysis method can change the application of the current to the electrodes and the resulting outcome.[4][5] This varies from direct current (DC) electrolysis, which only allows the variation of one value, the voltage applied. By utilising conventional pulse width modulation (PMW), multiple dependent variables can be altered, including the type of waveform, typically a rectangular pulse wave, the duty cycle, and the frequency. Currently, there has been a focus on theoretical and experimental research into PDC electrolysis in terms of the electrolysis of water to produce hydrogen. Past research has demonstrated that there is a possibility it can result in a higher electrical efficiency in comparison to DC electrolysis.[5] This would allow electrolysis procedures to produce greater volumes of hydrogen with a reduced electrical energy consumption.[4] Although theoretical research has made large promise for the efficiencies and benefits of utilising pulse electrolysis, it has many contradictions including a common issue that it is difficult to replicate the successes of patents experimentally and produces its own negative effects on the electrolyser.[5]
PDC electrolysis is not only confined to the electrolysis of water. Uses in industry such as electroplating and electrocrystallisation are also undergoing research due to the wider range of properties that can be achieved.[6]
The various and alterable effects of using intermittent pulses in PDC electrolysis has resulted in an area of interest that could benefit industry. However, as it is still being researched and has produced conflicting results, a consistent and reliable answer to how dependent electrolysis efficiency is on the properties of an electrical pulse has not been determined,[4] hence, other forms of electrolysis such as polymer electrolyte membrane and alkaline water electrolysis are being used in industry.
- ^ "Pulse electrolysis of alkaline solutions as highly efficient method of production of hydrogen/oxygen gas mixtures". ResearchGate. Retrieved 2019-05-31.
- ^ Morita, K.; Furuya, Etuo. (1994-07-01). "Pulse electrolysis within a solution boundary layer to minimize convective effects". Analytical Chemistry. 66 (13). U.S.: American Chemical Society: 2197–2199. doi:10.1021/ac00085a042.
- ^ Kireev, S. Yu. (2017-03-01). "Intensification of processes of electrodeposition of metals by use of various modes of pulse electrolysis". Inorganic Materials: Applied Research. 8 (2). Springer Science+Business Media: 203–210. doi:10.1134/S2075113317020095. S2CID 99479894.
- ^ a b c Poláčik, Ján; Pospíšil, Jiří (2016-10-01). "Some Aspects of PDC Electrolysis". Technological Engineering. 13 (1): 33–34. Bibcode:2016TeEng..13...33P. doi:10.2478/teen-2016-0011. ISSN 2451-3156. S2CID 99125043.
- ^ a b c Shaaban, Aly H. (1993). "Water Electrolysis and Pulsed Direct Current". Journal of the Electrochemical Society. 140 (10): 2863–2867. Bibcode:1993JElS..140.2863S. doi:10.1149/1.2220923.
- ^ Ibl, N.; Puippe, J.Cl.; Angerer, H. (1978). "Electrocrystallization in pulse electrolysis". Surface Technology. 6 (4): 287–300. doi:10.1016/0376-4583(78)90044-4.