 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /rəˈbɛprəˌzɔːl/ |
| Trade names | Aciphex, Pariet, Rafron, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699060 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Proton pump inhibitor[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 52%[2] |
| Protein binding | 96.3%[3] |
| Metabolism | CYP2C19 and CYP3A4 in the liver[2] |
| Metabolites | thioether carboyxlic acid metabolite, thioether glucuronide metabolite, sulfone metabolite[3] |
| Elimination half-life | ~1 hour[2] |
| Excretion | 90% via kidney as metabolites[4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.408 |
| Chemical and physical data | |
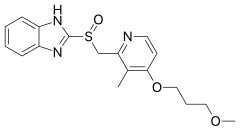
| Formula | C18H21N3O3S |
| Molar mass | 359.44 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture[2] |
| |
| |
| (verify) | |
Rabeprazole, sold under the brand name Aciphex, among others, is a medication that decreases stomach acid.[6] It is used to treat peptic ulcer disease, gastroesophageal reflux disease, and excess stomach acid production such as in Zollinger–Ellison syndrome.[6] It may also be used in combination with other medications to treat Helicobacter pylori.[7] Effectiveness is similar to other proton pump inhibitors (PPIs).[8] It is taken by mouth.[6]
Common side effects include constipation, feeling weak, and throat inflammation.[6] Serious side effects may include osteoporosis, low blood magnesium, Clostridioides difficile infection, and pneumonia.[6] Use in pregnancy and breastfeeding is of unclear safety.[1] It works by blocking H+/K+-ATPase in the parietal cells of the stomach.[6]
Rabeprazole was patented in 1986, and approved for medical use in 1997.[9] It is available as a generic medication.[7] In 2017, it was the 288th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[10]
- ^ a b c "Rabeprazole Use During Pregnancy". Drugs.com. 25 June 2018. Retrieved 10 January 2020.
- ^ a b c d e Cite error: The named reference
Marelli Review 2012was invoked but never defined (see the help page). - ^ a b Langtry HD, Markham A (October 1999). "Rabeprazole: a review of its use in acid-related gastrointestinal disorders". Drugs. 58 (4): 725–742. doi:10.2165/00003495-199958040-00014. PMID 10551440. S2CID 195691083.
- ^ "Rabeprazole". PubChem. NCBI. Retrieved 10 January 2020.
- ^ "Rabeprazole". PubChem. U.S. National Library of Medicine. Retrieved 10 January 2020.
- ^ a b c d e f "Rabeprazole Sodium Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 82. ISBN 9780857113382.
- ^ "[99] Comparative effectiveness of proton pump inhibitors". Therapeutics Initiative. 28 June 2016. Retrieved 14 July 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 445. ISBN 9783527607495.
- ^ "Rabeprazole - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.