 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| RaF2 | |
| Molar mass | 263.8214 g/mol[1] |
| Appearance | White cubic crystals[1] |
| Density | 6.7 g/cm3[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly radioactive and toxic |
| GHS labelling: | |

| |
| H350 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
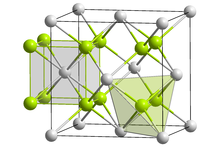
Radium fluoride is an inorganic compound with a chemical formula of RaF2. This salt, like all radium compounds, is highly radioactive. It can be coprecipitated with lanthanide fluorides.[2] Radium fluoride has the same crystal form as calcium fluoride (fluorite).[citation needed] However, calculations suggest that radium fluoride vapor consists of RaF2 molecules, with a bond angle of 118°, due to substantial covalent interaction within the molecule.[3]
- ^ a b c "Radium fluoride | 20610-49-5".
- ^ US 1655184, Hahn, Otto, "Radium preparation and process of making same", published 1928-01-03
- ^ Lee, Edmond P. F.; Soldán, Pavel; Wright, Timothy G. (2001-11-01). "The Heaviest Group 2 Difluoride, RaF 2 : Geometry and Ionization Energy". Inorganic Chemistry. 40 (23): 5979–5984. doi:10.1021/ic010538l. ISSN 0020-1669.