
| |
| |
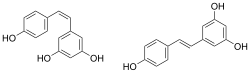 Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-[(E)-2-(4-Hydroxyphenyl)ethen-1-yl]benzene-1,3-diol | |
| Other names
trans-3,5,4′-Trihydroxystilbene;
3,4′,5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.386 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | white powder with slight yellow cast |
| Melting point | 261 to 263 °C (502 to 505 °F; 534 to 536 K)[2] |
| Solubility in water | 0.03 g/L |
| Solubility in DMSO | 16 g/L |
| Solubility in ethanol | 50 g/L |
| UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
| GHS labelling:[5] | |

| |
| Warning | |
| H319 | |
| P264, P280, P305+P351+P338, P337+P313 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
23.2 μM (5.29 g)[4] |
| Safety data sheet (SDS) | Fisher Scientific[2] Sigma Aldrich[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol or polyphenol and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi.[6][7] Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.[8][9]
Although commonly used as a dietary supplement and studied in laboratory models of human diseases,[10] there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease.[11][12]
- ^ a b Camont, Laurent; Cottart, Charles-Henry; Rhayem, Yara; et al. (February 2009). "Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions". Anal. Chim. Acta. 634 (1): 121–128. Bibcode:2009AcAC..634..121C. doi:10.1016/j.aca.2008.12.003. PMID 19154820.
- ^ a b "Resveratrol MSDS on Fisher Scientific website". Archived from the original on 2012-11-03. Retrieved 2012-03-06.
- ^ Resveratrol MSDS on www.sigmaaldrich.com
- ^ Bechmann LP, Zahn D, Gieseler RK, et al. (June 2009). "Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells". Hepatology Research. 39 (6): 601–608. doi:10.1111/j.1872-034X.2008.00485.x. PMC 2893585. PMID 19207580.
- ^ GHS: Sigma-Aldrich R5010
- ^ "Resveratrol". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 11 June 2015. Retrieved 26 August 2019.
- ^ Fremont, Lucie (January 2000). "Biological Effects of Resveratrol". Life Sciences. 66 (8): 663–673. doi:10.1016/S0024-3205(99)00410-5. PMID 10680575.
- ^ Jasiński M, Jasińska L, Ogrodowczyk M (August 2013). "Resveratrol in prostate diseases – a short review". Central European Journal of Urology. 66 (2): 144–149. doi:10.5173/ceju.2013.02.art8. PMC 3936154. PMID 24579014.
- ^ Cite error: The named reference
pewas invoked but never defined (see the help page). - ^ "Resveratrol". MedlinePlus. 1 April 2019. Retrieved 22 September 2019.
- ^ Cite error: The named reference
pmid21698226was invoked but never defined (see the help page). - ^ Sahebkar A, Serban C, Ursoniu S, et al. (2015). "Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors – Results from a systematic review and meta-analysis of randomized controlled trials". International Journal of Cardiology. 189: 47–55. doi:10.1016/j.ijcard.2015.04.008. PMID 25885871.