 | |
| Clinical data | |
|---|---|
| Trade names | Trobalt, Potiga |
| Other names | D-23129, ezogabine (USAN US) |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a612028 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60% |
| Protein binding | 60–80% |
| Metabolism | Liver glucuronidation and acetylation. CYP not involved |
| Elimination half-life | 8 hours (mean), range: 7–11 hours[1] |
| Excretion | Kidney (84%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.123 |
| Chemical and physical data | |
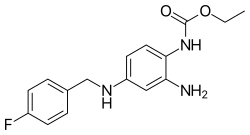
| Formula | C16H18FN3O2 |
| Molar mass | 303.337 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients.[2] The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.[3][4]
Retigabine works primarily as a potassium channel opener—that is, by activating a certain family of voltage-gated potassium channels in the brain.[5][6][7] This mechanism of action is unique among antiepileptic drugs, and may hold promise for the treatment of other neurologic conditions, including tinnitus, migraine and neuropathic pain. The manufacturer withdrew retigabine from clinical use in 2017.
- ^ Ferron GM, Paul J, Fruncillo R, Richards L, Knebel N, Getsy J, Troy S (February 2002). "Multiple-dose, linear, dose-proportional pharmacokinetics of retigabine in healthy volunteers". Journal of Clinical Pharmacology. 42 (2): 175–182. doi:10.1177/00912700222011210. PMID 11831540. S2CID 5568963.
- ^ "POTIGA (ezogabine) Tablets, CV. Full Prescribing Information" (PDF). GlaxoSmithKline and Valeant Pharmaceuticals. Retrieved 4 June 2014.
- ^ https://assets.publishing.service.gov.uk/media/57fe4b6640f0b6713800000c/Trobalt_letter.pdf [bare URL PDF]
- ^ "Epilepsy drug Trobalt (retigabine) to be discontinued". epilepsysociety.org.uk. 14 September 2016.
- ^ Rundfeldt C (October 1997). "The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells". European Journal of Pharmacology. 336 (2–3): 243–249. doi:10.1016/S0014-2999(97)01249-1. PMID 9384239.
- ^ Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA (August 2000). "Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine". Molecular Pharmacology. 58 (2): 253–262. doi:10.1124/mol.58.2.253. PMID 10908292. S2CID 11112809.
- ^ Rogawski MA, Bazil CW (July 2008). "New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels". Current Neurology and Neuroscience Reports. 8 (4): 345–352. doi:10.1007/s11910-008-0053-7. PMC 2587091. PMID 18590620.