 | |
| Clinical data | |
|---|---|
| Trade names | Vexol |
| Other names | Trimexolone; Org 6216; 11β-Hydroxy-16α,17α,21-trimethylpregna-1,4-dien-3,20-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606003 |
| Routes of administration | Eye drops |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | estimated 1–2 hours |
| Excretion | >80% faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.211.227 |
| Chemical and physical data | |
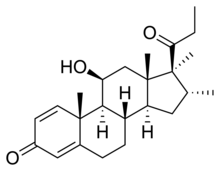
| Formula | C24H34O3 |
| Molar mass | 370.533 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rimexolone is a glucocorticoid steroid used to treat inflammation in the eye.[1] It is marketed as a 1% eye drop suspension under the trade name Vexol by Alcon Laboratories, but was discontinued in the US and other countries.[2][3]
- ^ Kavuncu S, Horoz H, Ardagil A, Erbil HH (August 2008). "Rimexolone 1% versus prednisolone acetate in preventing early postoperative inflammation after cataract surgery". Int Ophthalmol. 28 (4): 281–5. doi:10.1007/s10792-007-9131-0. PMID 17762913. S2CID 20772101.
- ^ Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Vexol 1% (10 mg/ml)-Augentropfensuspension.
- ^ Drugs.com: Monograph.