| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ruthenium(VIII) oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.039.815 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
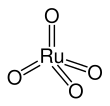
| RuO4 | |||
| Molar mass | 165.07 g/mol | ||
| Appearance | yellow easily melting solid | ||
| Odor | pungent | ||
| Density | 3.29 g/cm3 | ||
| Boiling point | 129.6[1] °C (265.3 °F; 402.8 K) | ||
| 2% w/v at 20 °C | |||
| Solubility in other solvents | Soluble in Carbon tetrachloride Chloroform | ||
| Structure | |||
| tetrahedral | |||
| zero | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | external MSDS sheet | ||
| Related compounds | |||
Related compounds
|
Ruthenium dioxide Ruthenium trichloride Osmium tetroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature.[2] It has the odor of ozone.[3] Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.[4]
- ^ Koda, Yoshio (1986). "Boiling Points and Ideal Solutions of Ruthenium and Osmium Tetraoxides". Journal of the Chemical Society, Chemical Communications. 1986 (17): 1347–1348. doi:10.1039/C39860001347.
- ^ H. L. Grube (1963). "Ruthenium (VIII) Oxide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY: Academic Press. pp. 1599–1600.
- ^ Cite error: The named reference
Backman_2004was invoked but never defined (see the help page). - ^ Martín, V. S.; Palazón, J. M.; Rodríguez, C. M.; Nevill, C. R. (2006). "Ruthenium(VIII) Oxide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rr009.pub2. ISBN 978-0471936237.